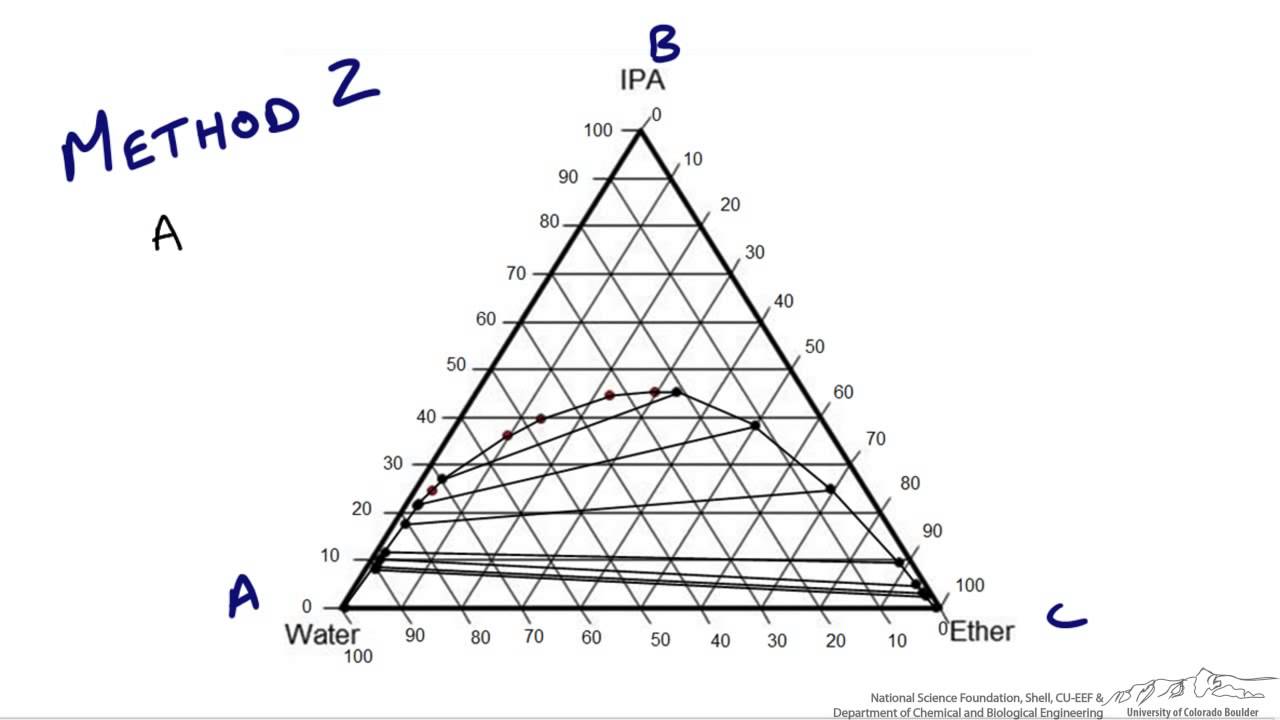
38 ternary phase diagram tie lines
Interpolating Tie Lines on a Ternary Diagram. mirror. Lever Rule (Interactive) Lever Rule Derivation. mirror. Multicondensable Species in VLE. mirror. Partial Pressure: Immiscible Liquids. mirror . Phase Equilibrium: Txy Diagram. mirror. Plotting a Ternary Phase Diagram. mirror. Pxy Diagram for VLE (Simulation) mirror. Raoult's Law (Water as Condensable Component) …
Isopleth through hypothetical ternary phase diagram at a constant . 40% . C. adapted from ref 10.1. SPB Line. The single-phase boundary line is found on any section that . contains a single-phase region. The line is what its name implies. It is the boundary line around that single-phase region. It can be used, for example, to determine compositions and temperatures where an …
by N Bezlyepkina · Cited by 95 — Phase diagram and tie-line determination for the ternary ... FIGURE S1 Phase diagram of the ternary mixture DOPC/SM/Chol at (23±1)°C. Data available in the ...12 pages
Ternary phase diagram tie lines
Knowing the boundaries of the regions in the phase diagram is not sufficient to characterize the composition of domains in a multicomponent vesicle. The domain composition is defined by the tie lines in the coexistence region. Locating the tie lines is challenging because the coexisting phases in the bilayer membrane cannot be phys-
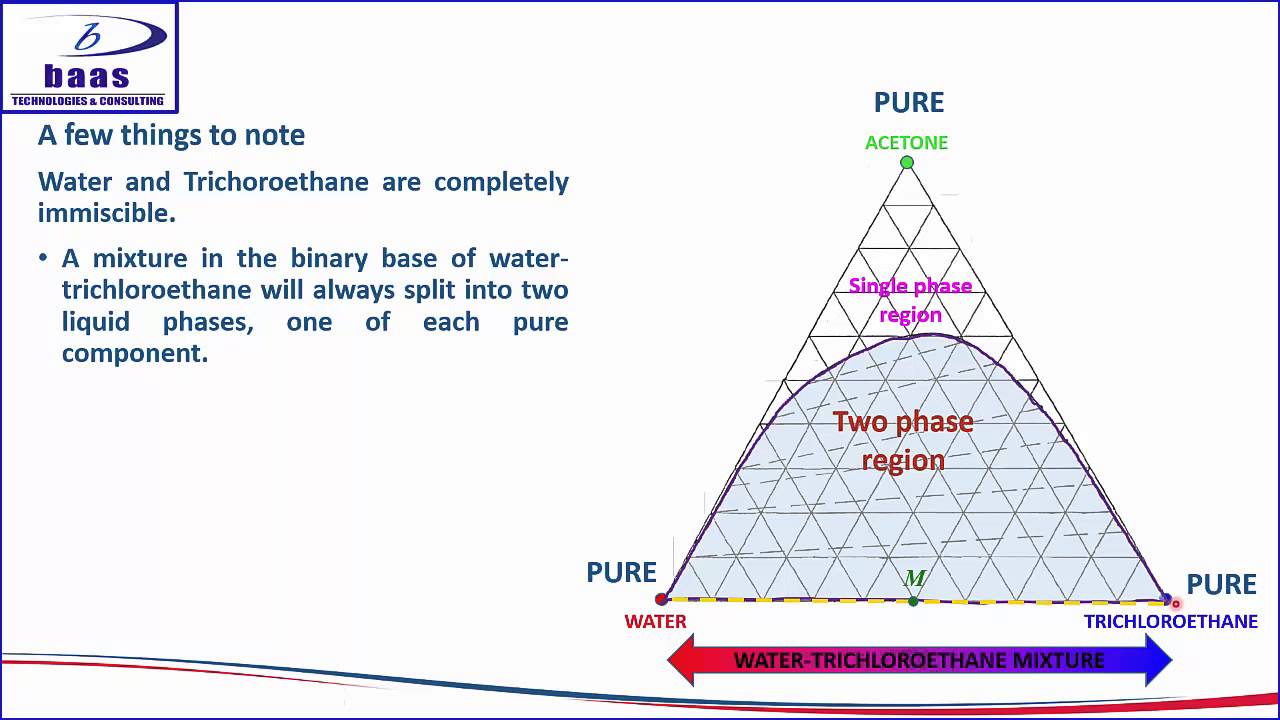
An example of a phase diagram for a ternary alloy is shown for a fixed T and P below. Phase diagrams for binary systems ternary phase diagram of Ni-Cr-Fe. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 11 Isomorphous system - complete solid solubility of the two components (both in the liquid and solid phases). Binary Isomorphous Systems (I) …
Ternary phase diagrams are used to represent all possible mixtures of three solvents [1]; they are described in Chapter 3.Here, we shall indicate how they should be used to minimize the solvent consumption. Figure 2.1 (top) shows the methanol–chloroform–water ternary phase diagram with the tie-lines in the biphasic domain. Five particular compositions are shown in the …
Ternary phase diagram tie lines.
The process for calculating the percentage of liq vs. two crystallizing solids in a ternary eutectic system is supposed to be modeled geometrically by an isothermal triangle whose centroid is the bulk composition of the system. Here is an image to help clarify what I’m asking. I’m asking about the ratio between the two line segments liq-a and a-m in the linked image. Image: https://imgur.com/a/zfTicdT If you draw a line from the point representing a liquid’s composition along the cotectic t...
The simplest phase diagrams are pressure–temperature diagrams of a single simple substance, such as water.The axes correspond to the pressure and temperature.The phase diagram shows, in pressure–temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas.. The curves on the phase diagram show the points where the …
29.10.2020 · VLE-Calc.com has been renewed − phase diagrams and distillation calculation After 5 years of development a completely rewritten and redesigned program is released. More materials and more types of charts are built-in, and an easier-to-use interface (which is more mobile-friendly) has been formed.
Mar 15, 2020 · Then, what is a tie line in ternary phase diagram? Tie lines connect compositions of liquid and vapor phases in equilibrium. Any mixture with an overall composition along a tie line gives the same liquid and vapor compositions. Only the amounts of liquid and vapor change as the overall composition changes from the liquid side of the binodal curve to the vapor side.
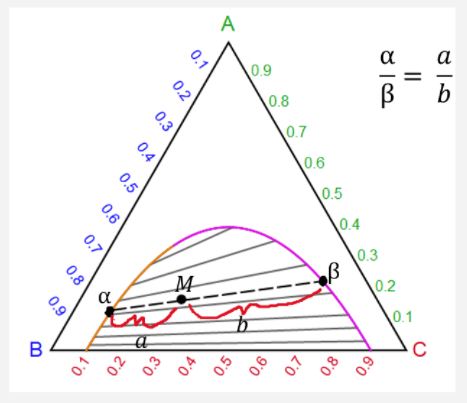
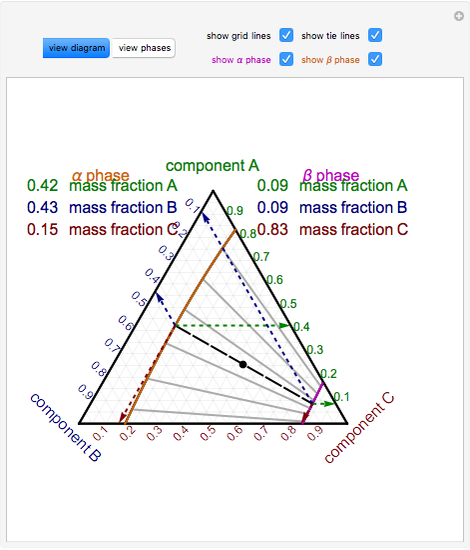
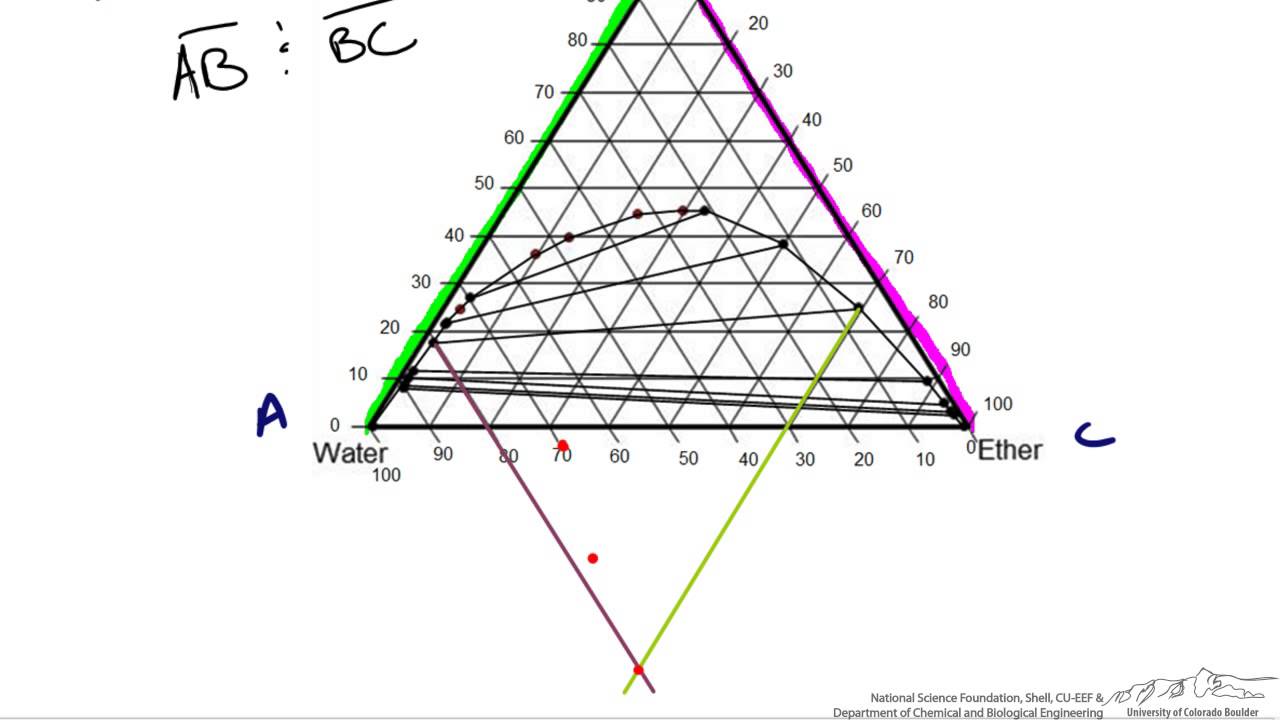
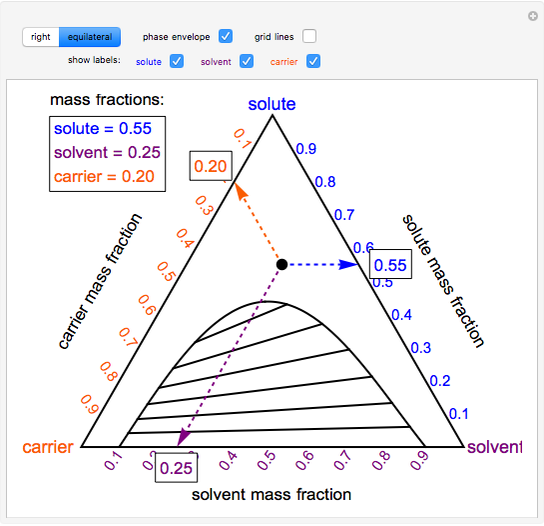
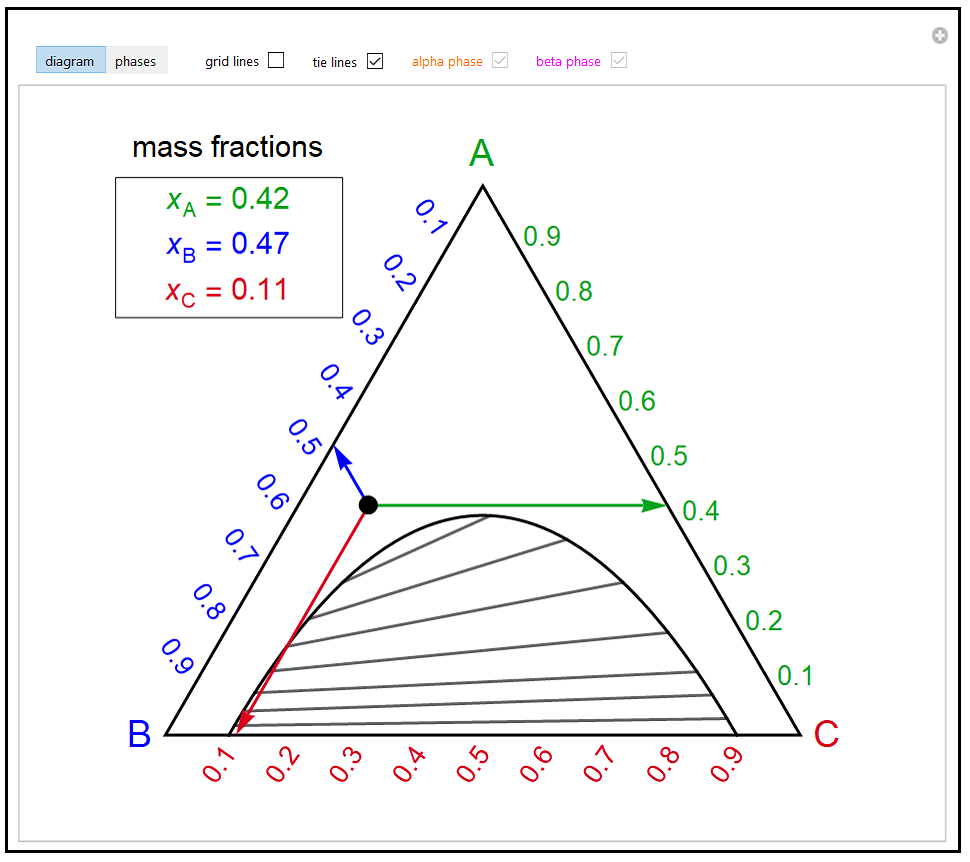
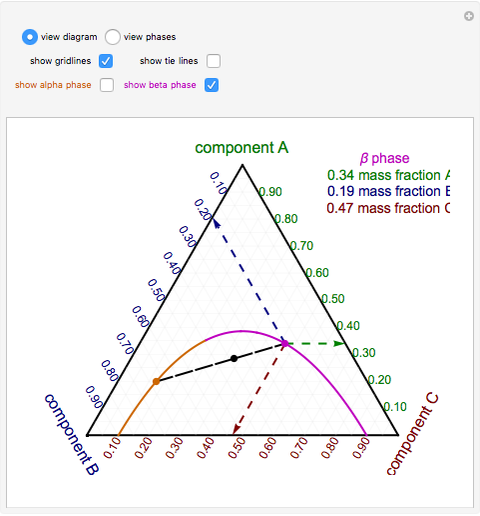
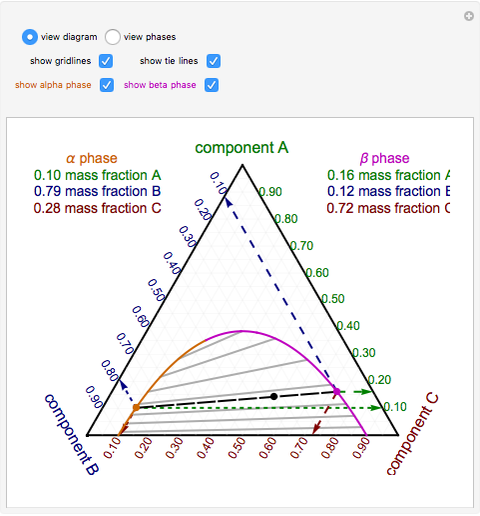
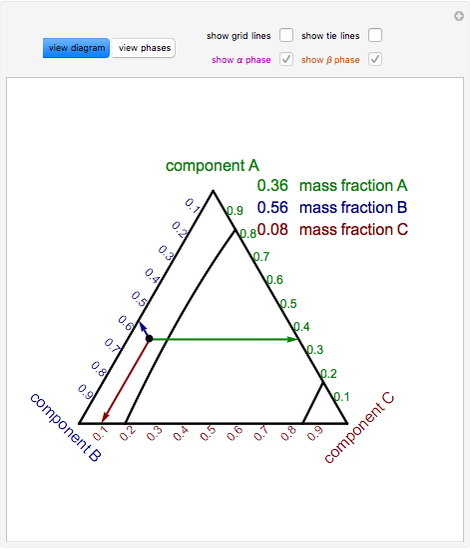
Phase behavior of mixtures containing three components is represented conveniently on a triangular diagram such as those shown in Fig. 1. Such diagrams are based on the property of equilateral triangles that the sum of the perpendicular distances from any point to each side of the diagram is a constant equal to the length of any of the sides. Thus, the composition of a point in the interior of the triangle can be calculated as ....................(1) where ....................(2) Several other useful properties of triangular diagrams are a consequence of this fact. For mixtures along any line parallel to a side of the diagram, the fraction of the component of the corner opposite to that side is constant (Fig. 1b). In addition, mixtures lying on any line connecting a corner with the opposite side contain a constant ratio of the components at the ends of the side (Fig. 1c). Finally, mixtures of any two compositions, such as A and B in Fig. 1d, lie on a straight line connecting the two...
Jan 24, 2020 · Keeping this in view, what is a tie line in phase diagram? An isothermal (constant temperature) line through the alloy's position on the phase diagram when it is in a two phase field, intersecting the two adjacent solubility curves, is called a tie line (yes, that's the horizontal yellow line on the diagram). One may also ask, what is a ternary system?
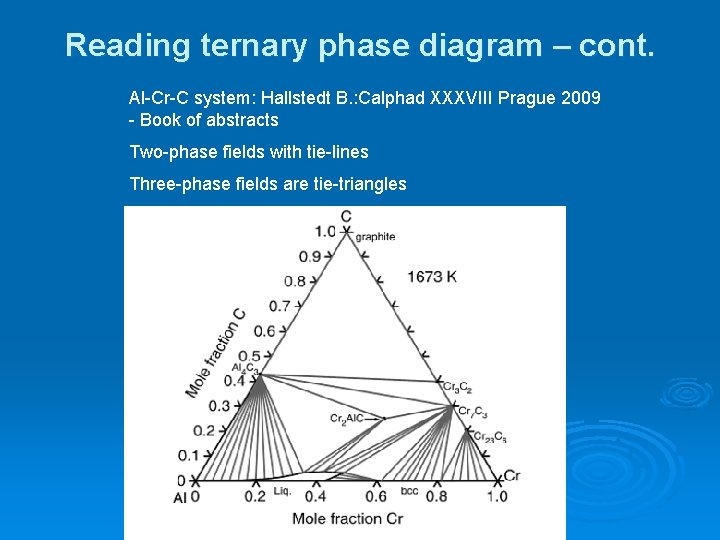
The Ti-Si-N-O quaternary phase diagram • Entire phase diagram can be calculated by taking into account all possible combinations of reactions and products • 4 ternary diagrams of Ti-Si-N, Ti-N-O, Ti-Si-O and Si-N-O were evaluated • additional quaternary tie lines from TiN to SiO 2 and Si 2N 2O A.S.Bhansali, et al., J.Appl.Phys. 68(3 ...
Locate point on the ternary phase diagram. It will be on the equilibrium curve. Draw a straight line from to and extend to find the location of on the equilibrium curve. On a fresh copy of the graph, with plenty of blank space on each side of the diagram, note the location of points , , and (specified/selected) and (determined in step 3).
Analysing ternary microstructures. • Ensure phases >3µm (interaction volume, which ↓ with ↓ kV • At least 5 measuremnts on different phases • (but need higher kV to excite necessary peaks..) • Overall should lie on tie line of 2 phases, else. – Phase missing – At least one inaccurate result – suspect smallest!
In a two phase equilibrium, a tieline represents an iso-chemical potential line at fixed (T,p). The variance of the ternary system is equal to 1. Since all the ...10 answers · 0 votes: You might take a look at this video: (not mine but I think it illustrates the idea ...
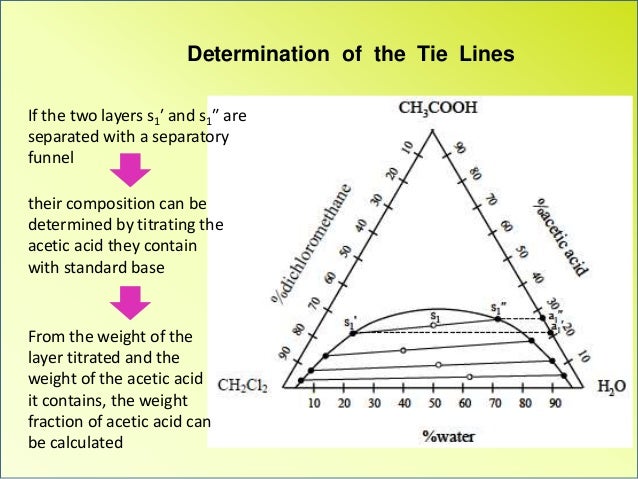
Tie Lines in Phase Diagrams for Ternary Liquid Systems. Most laboratory programs in physical chemistry at the undergraduate level include an exercise in the construc- tion of a phase diagram, on triangular coordinate paper, for a ternary liquid system. The experimental method used for this exercise is almost always a titration method in which the endpoint is the appearance (or disappear- ance) of turbidity when the system changes from one phase to two phases (or vice versa).
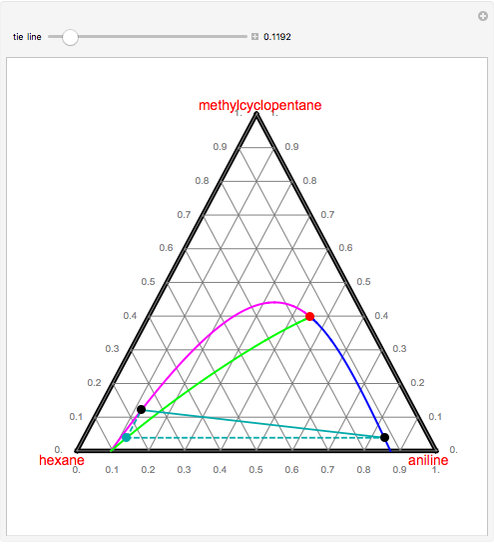
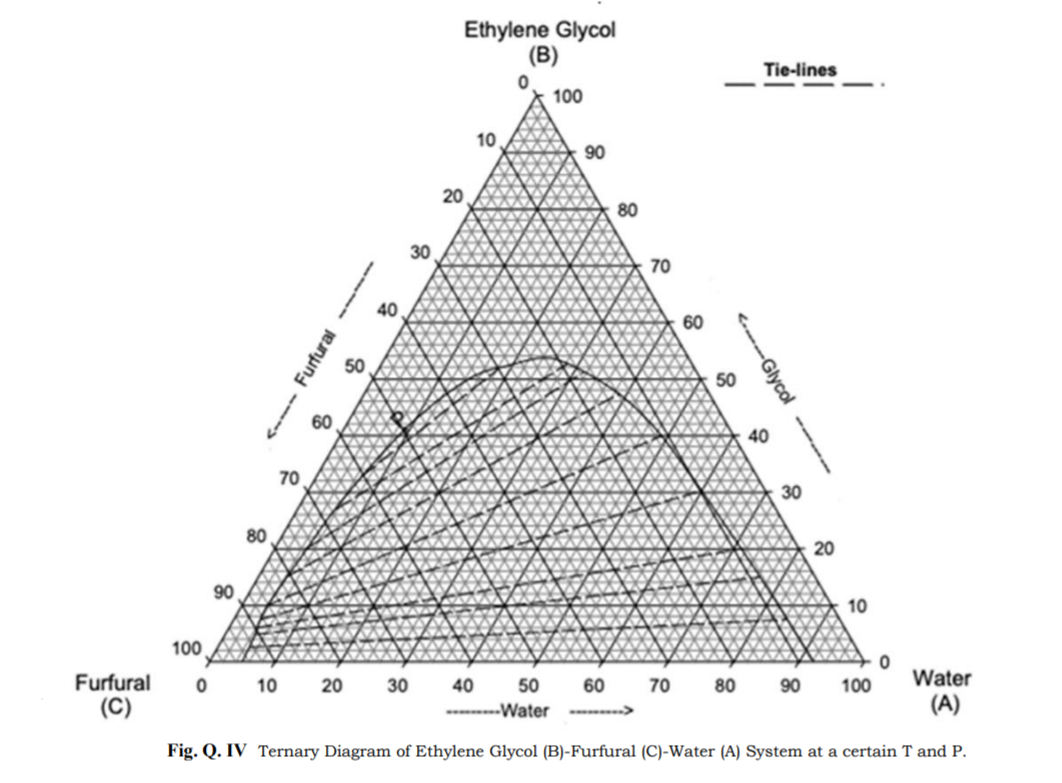
by SN Csernica · 2011 · Cited by 29 — Tie lines A−F represent data points in which the top phase consisted of biodiesel while the bottom phase contained the glycerol. Tie lines G−I ...
I am looking for (preferably free) software for constructing ternary phase diagrams and plotting tie lines, phases etc.... Any suggestions would be welcome.
09.04.1997 · Note on Tie Lines. Rarely do you have all the equilibrium tie lines you want. It is thus good to know that there is a fairly easy way of generating additional lines. To do this, you construct a "conjugate curve" from the existing tie lines. Take each endpoint and draw a line from it downward, perpendicular to base of the triangle. The ...




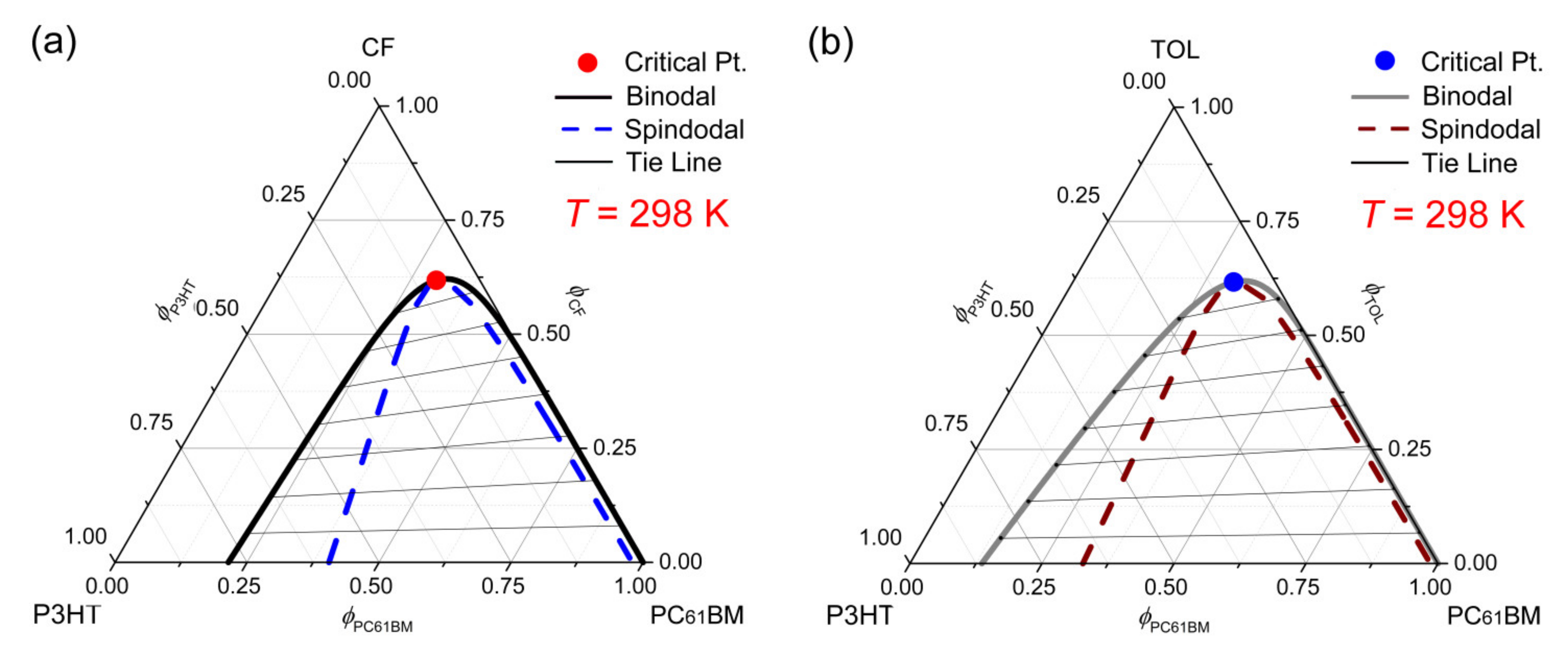







![PDF] Phase diagram and tie-line determination for the ternary ...](https://d3i71xaburhd42.cloudfront.net/725aa4be1c0fd7cf111062971888c2215e4ae6b7/6-Figure5-1.png)






0 Response to "38 ternary phase diagram tie lines"
Post a Comment