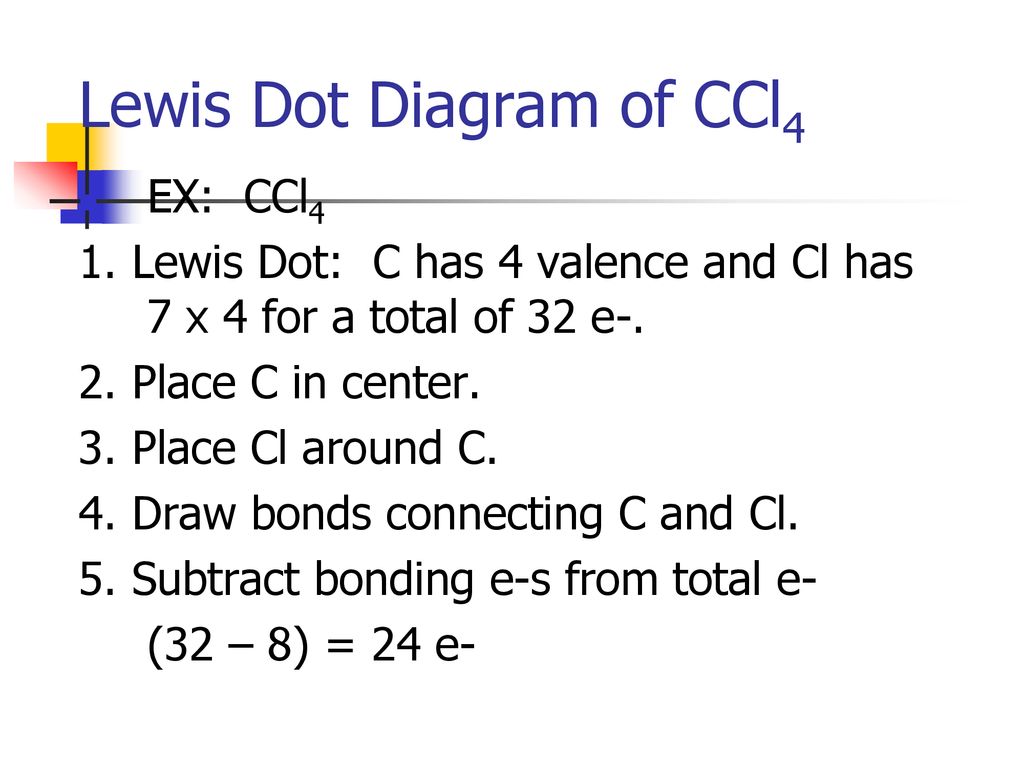
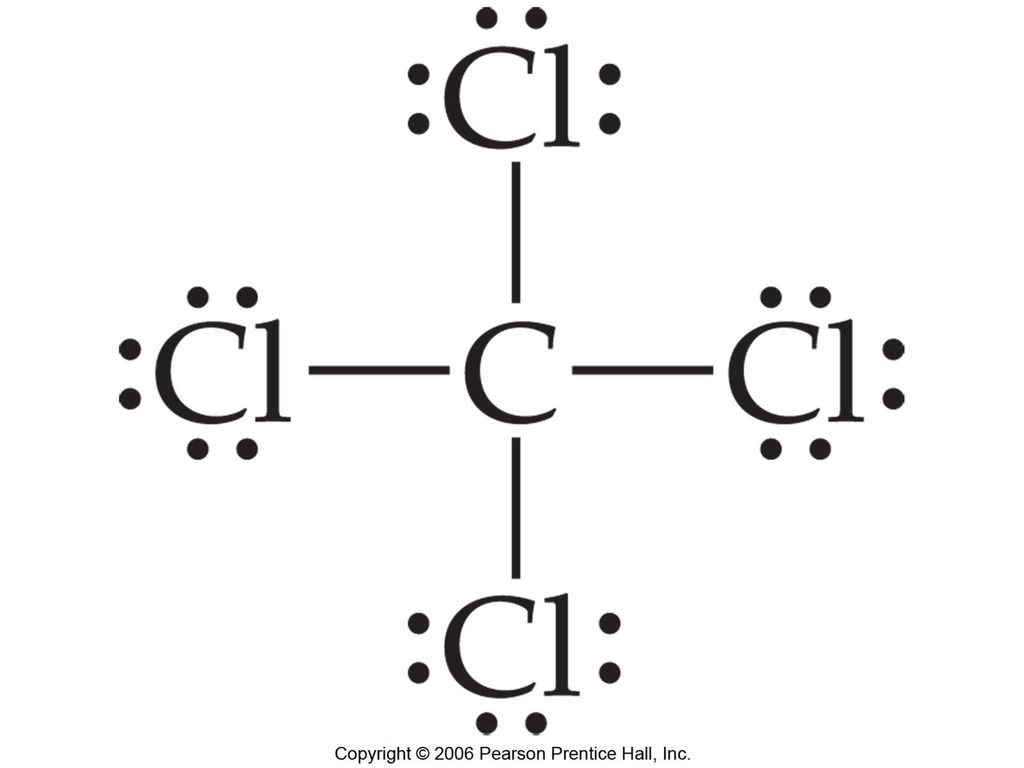
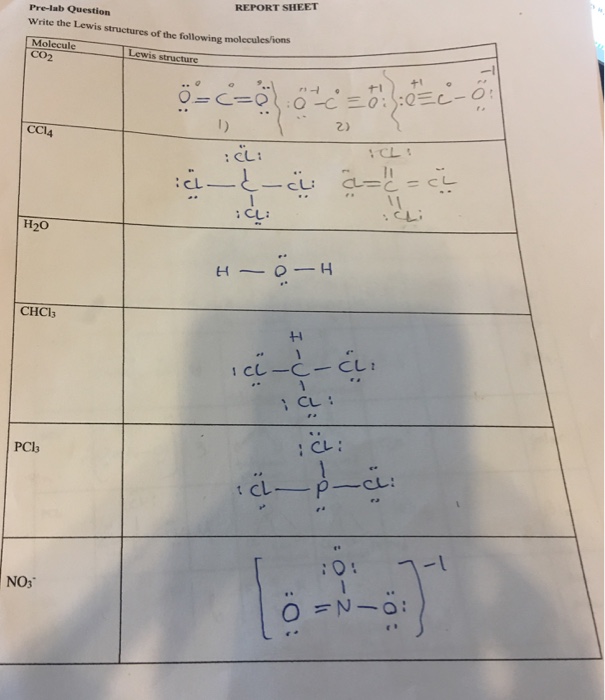
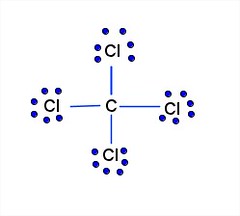
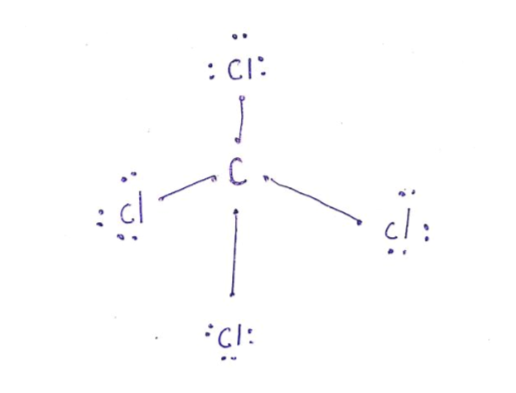
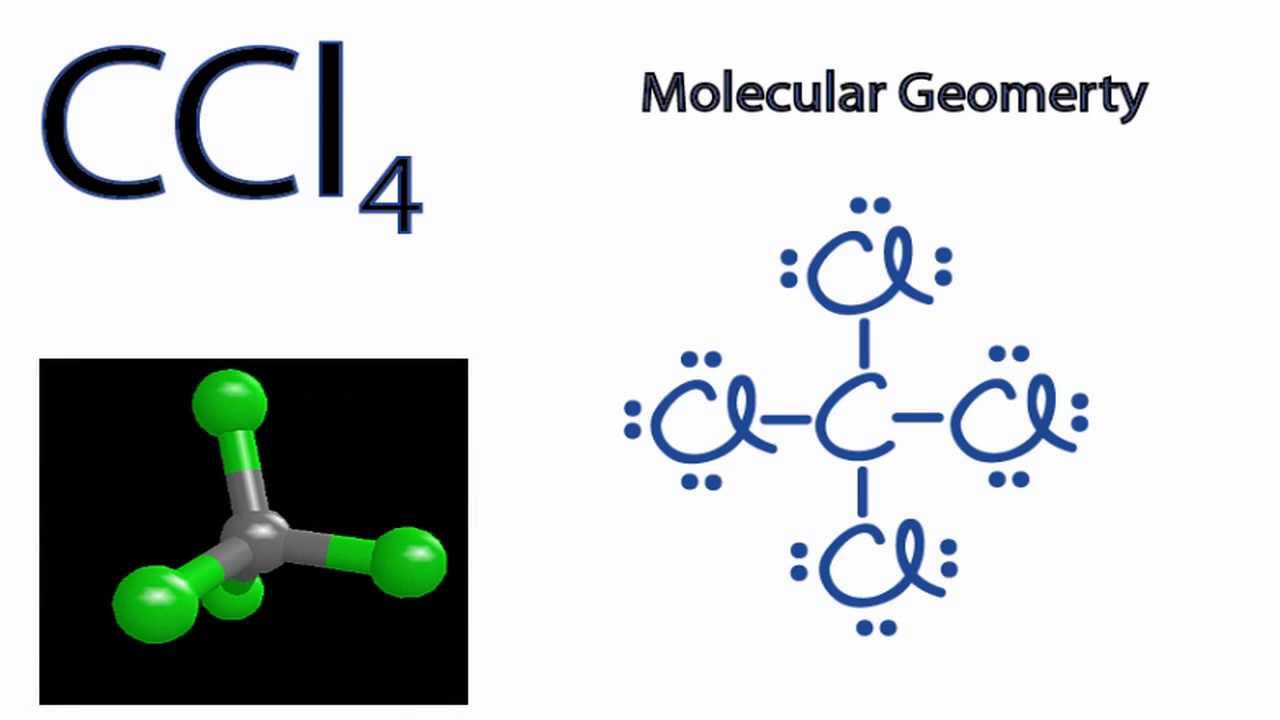
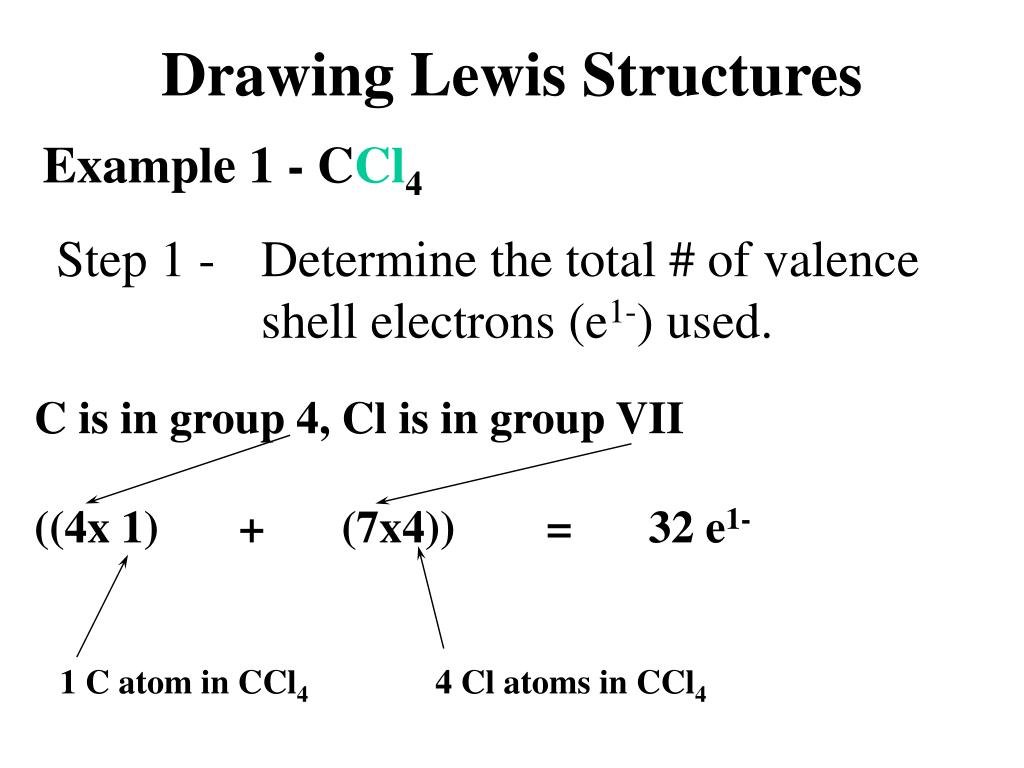
38 lewis dot diagram for ccl4
I quickly take you through how to draw the Lewis Structure of CCl4 (Carbon TetraChloride). I also go over hybridization, shape and bond angle. A CCL4 Lewis structure is a diagram that represents the electron configuration of covalently bonded compounds.Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound.. Carbon tetrachloride (CCl 4) is a covalently bonded compound composed of a central carbon surrounded by 4 chlorine atoms in a tetrahedral ...
2:05How to Draw a Lewis Structure for CCl4? Lewis Structure: ...15 Jun 2019 · Uploaded by Dr. Masi

Lewis dot diagram for ccl4
1:50Hey Guys,In this video we are going to learn about the Lewis structure of CCl4. It is a chemical formula for ...8 Feb 2021 · Uploaded by Geometry of Molecules In the carbon tetrachloride molecule, four chlorine atoms are positioned symmetrically as corners in a tetrahedral configuration joined to a central carbon atom ...22 Sep 2010 · Uploaded by kentchemistry.com Transcribed image text: Draw the Lewis dot structure for CCl_4 and indicate the shape of the molecule Draw the Lewis dot structure for NH_3 and indicate the shape of the molecule Lewis dot structure: What is the O-N-O bond angle in the nitrate ion? What is the shape of a molecule with two bond charge clouds and two electron pair charge clouds? Linear Tetrahedral Bent Trigonal
Lewis dot diagram for ccl4. 10:02CCl4 Lewis Structure|| Lewis Structure for CCl4 (Carbon Tetrachloride)||Which is the correct Lewis structure ...16 Nov 2019 · Uploaded by Chemistry 360 1:226.03 Draw the Lewis structure for CCL4. 2,792 views2.7K views. May 24, 2016. 10. 2. Share. Save. 10 / 2 ...24 May 2016 · Uploaded by Allison Soult A CCL4 Lewis structure is a diagram that represents the electron configuration of covalently bonded compounds. Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound. A regular atom of carbon has 4 lone electrons in its outer shell. Question: Draw a Lewis dot structure for CCl4 including any lone pairs. This problem has been solved! See the answer See the answer See the answer done loading. Draw a Lewis dot structure for CCl4 including any lone pairs. Best Answer. This is the best answer based on feedback and ratings. Previous question Next question.
Lewis Dot Structure and Polarity of CCl4 (Carbon Tetrachloride) Carbon tetrachloride, also known as tetrachloromethane, is a compound containing carbon and chlorine. It is an inorganic compound that is non-flammable. This ScienceStruck post provides you with the Lewis dot structure diagram and the polarity of carbon tetrachloride. 1:47CCl4 Lewis Structure How to Draw the Dot Structure for CCl4 Carbon Tetachloride. Watch later. Share. Copy ...7 Mar 2019 · Uploaded by Alyssa Ellingson A step-by-step explanation of how to draw the CCl4 Lewis Dot Structure (Carbon tetrachloride). The Lewis structure for CCl4 is a commonly tested Lewis struc... Sticks or straight lines represent the bonds. While the dots represent the non-bonding electrons. Lewis theory is based on the octet rule, which states that an atom should have eight electrons in its outer shell to be stable. For the Lewis structure of CCl4 first, let’s calculate the total valence electrons.
CCl4 is also named carbon chloride, methane tetrachloride, benziform, and more. The liquid is not soluble in water and is non-combustible. The boiling point of CCl4 is 76.8 degrees Celcius and its melting point is -23.0 degrees Celcius. CCl4 will release toxic fumes like carbon monoxide. if it is led to decomposition. CCl4 lewis’s structure is made up of one carbon atom that is situated at the middle position and four chlorine atoms that are at the surrounding position. The total lone pair present in the CCl4 lewis dot structure is 12. Lone pairs of electrons do not involve in chemical bonds and it is represented as a dot in the lewis diagram. Transcribed image text: Draw the Lewis dot structure for CCl_4 and indicate the shape of the molecule Draw the Lewis dot structure for NH_3 and indicate the shape of the molecule Lewis dot structure: What is the O-N-O bond angle in the nitrate ion? What is the shape of a molecule with two bond charge clouds and two electron pair charge clouds? Linear Tetrahedral Bent Trigonal In the carbon tetrachloride molecule, four chlorine atoms are positioned symmetrically as corners in a tetrahedral configuration joined to a central carbon atom ...22 Sep 2010 · Uploaded by kentchemistry.com
1:50Hey Guys,In this video we are going to learn about the Lewis structure of CCl4. It is a chemical formula for ...8 Feb 2021 · Uploaded by Geometry of Molecules

Review Write The Electron Configuration For An Atom Of Carbon And Chlorine Determine The Empirical And Molecular Formula For A Compound Consisting Of Ppt Download
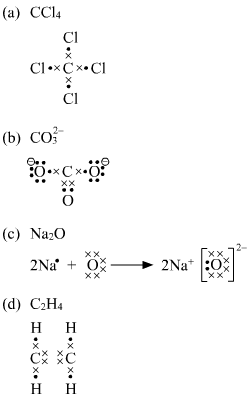
Draw The Lewis Structures Of The Following Ccl4 Co32 Na2o C2h4 Chemistry Chemical Bonding And Molecular Structure 8154075 Meritnation Com

Use The Vsepr Theory To Predict The Shape Of Carbon Tetrachloride Ccl4 A Tetrahedral B Bent C Trigonal Pyramidal D Trigonal Planar Study Com

48 Problem 4 If Atomic Mass Of Carbon Was Taken As 100 U Then What Would Be The Value Of Avogadro S Number
Give The Electron Dot Structure Of Ch3cl And C2h2 Sarthaks Econnect Largest Online Education Community



















0 Response to "38 lewis dot diagram for ccl4"
Post a Comment