39 bohr diagram for beryllium
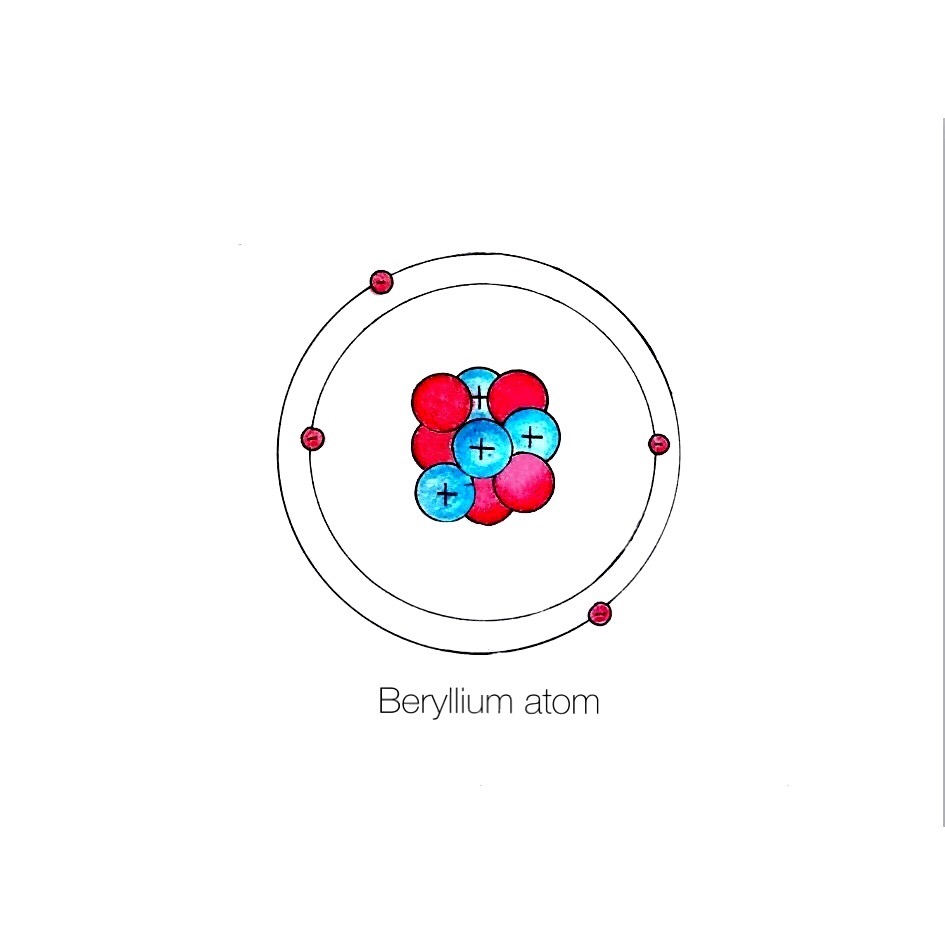
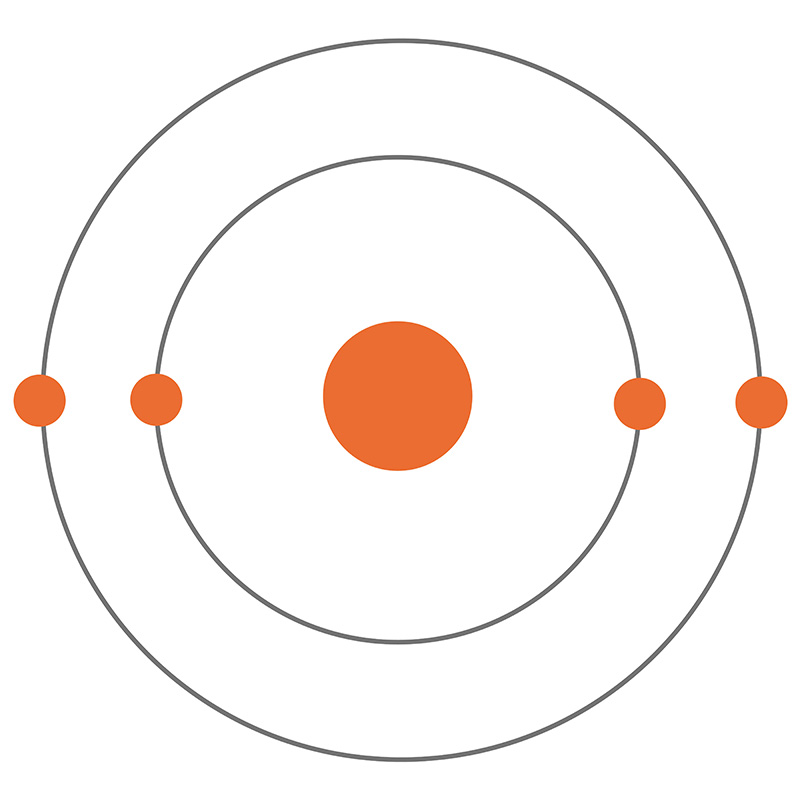
Bookmark the Beryllium bohr diagram Beryllium the the emerald house. A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. Beryllium has four electrons to balance the four protons. The 4 electrons are arranged with 2 electrons in the first orbital and 2 electrons in the second orbital. What is the symbol for beryllium? Be. How are Bohr and Lewis diagrams similar? A Lewis dot structure is like a simplified Bohr-Rutherford model.
Answer to Class Example - Bohr Electron Configuration Drawing for Beryllium after it has satisfied the Octet Rule in Lesson 1.4

Bohr diagram for beryllium
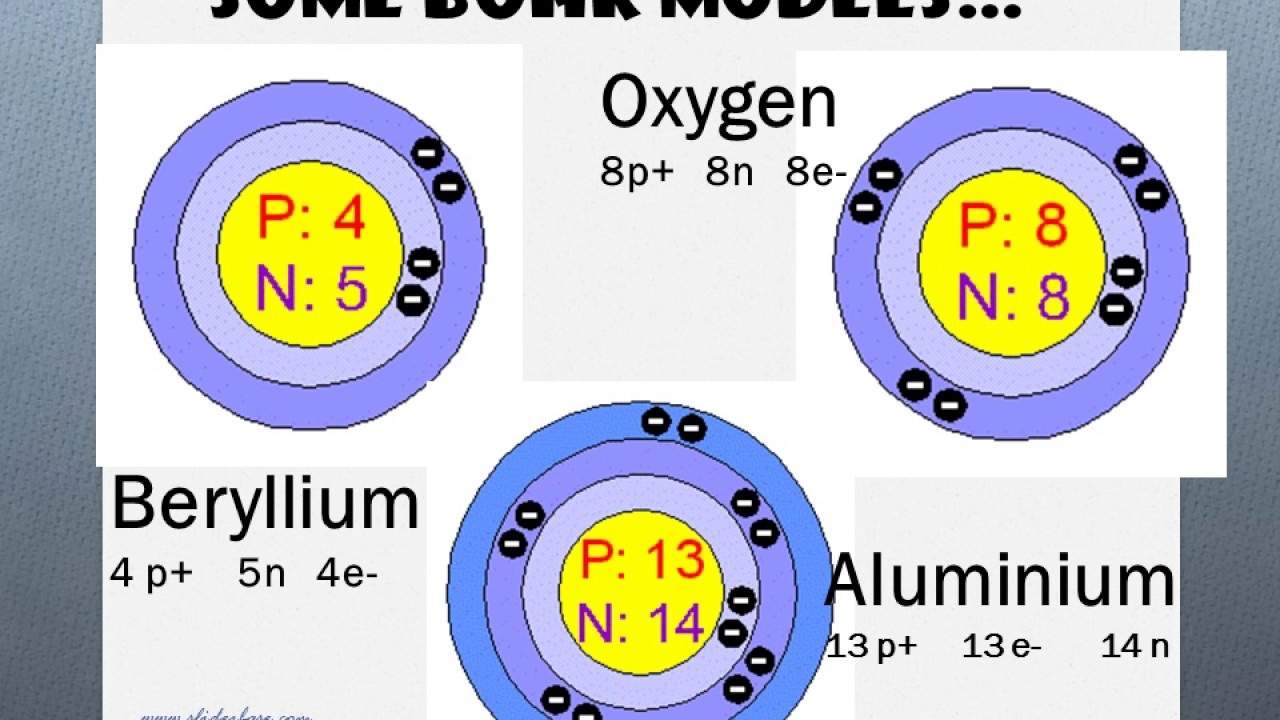
Beryllium Bohr model by Anthony Ragazzi - October 19, 2012. Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He Beryllium 2. Hydrogen 009 5. Sodium 1 8. Magnesium 11. Silicon . Science 9 Chapter 2 — Elements and the Periodic Table Identify the elements whose Bohr model diagrams are shown below. Write the names of the elements in the spaces provided. O O (f) (a) oo (c) O (d) (b) (c) (d) Name Use with textbook pages 64-67.
Bohr diagram for beryllium. Beryllium. 5 Answers. c. 0 . Draw a Bohr Model of Beryllium Draw a Bohr Model of Chlorine Activity. N- 12 neutrons.Name Period Date Bohr Model Diagrams. Beryllium is the fourth element listed on the periodic table. Beryllium?s atomic mass is 9 and its atomic number is 4. b. Answer the following additional questions. Beryllium oxide is a beryllium molecular entity consisting of beryllium (+2 oxidation state) and oxide in the ratio 1:1. In the solid state, BeO adopts the hexagonal wurtzite structure form while in the vapour phase, it is present as discrete diatomic covalent molecules. It has a role as a carcinogenic agent. The phosphorus bohr model has 3 shells because it has 15protons and 16 neutrons. Beryllium Atom Bohr model with proton, neutron and electron. 3d illustration ( is about 31, so 15protons = 16 neutrons the number of electrons is the same number of protons in an. Bohr Model Diagrams. It is the original image provided by the contributor. Bohr diagram is very interesting and easy to draw. Let's see How to draw a Bohr diagram for an atom? To draw the Bohr model of an atom, we should follow 4 or 5 basic steps. Find the number of protons, electrons, and neutrons of an atom. Draw the nucleus of an atom. Write the number of protons and neutrons at the center of the nucleus.
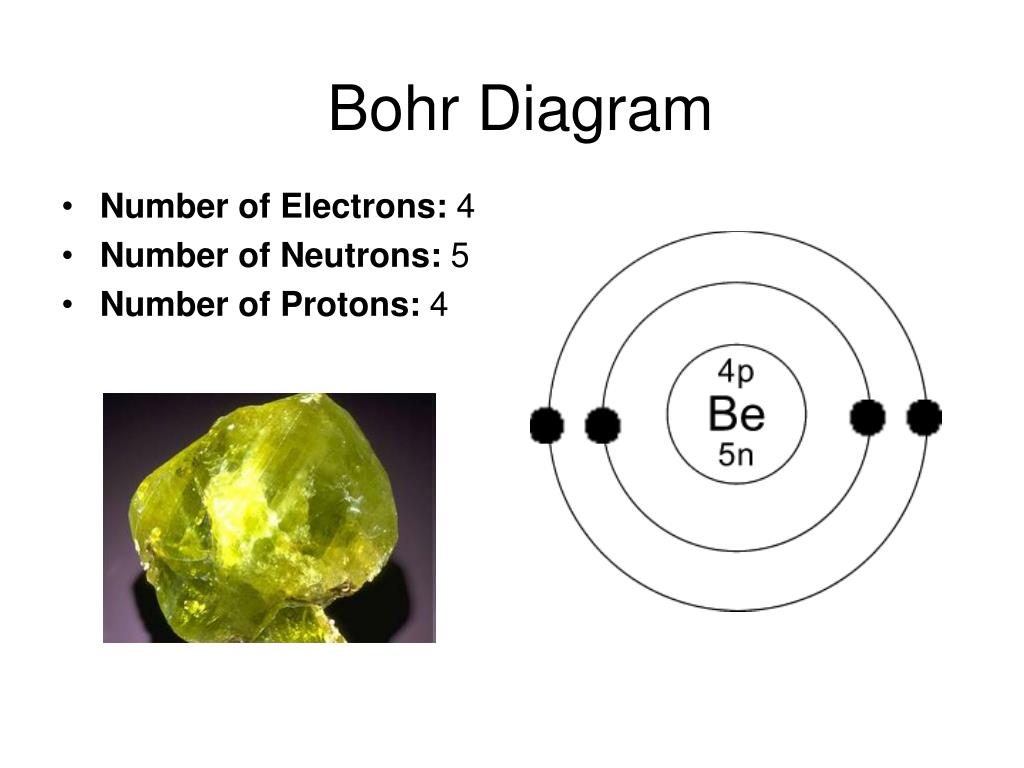
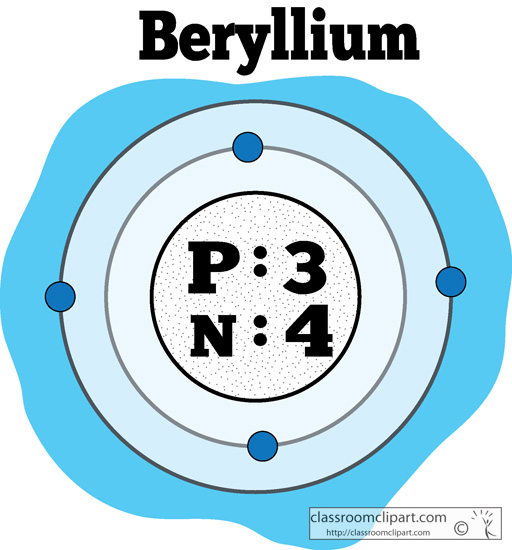
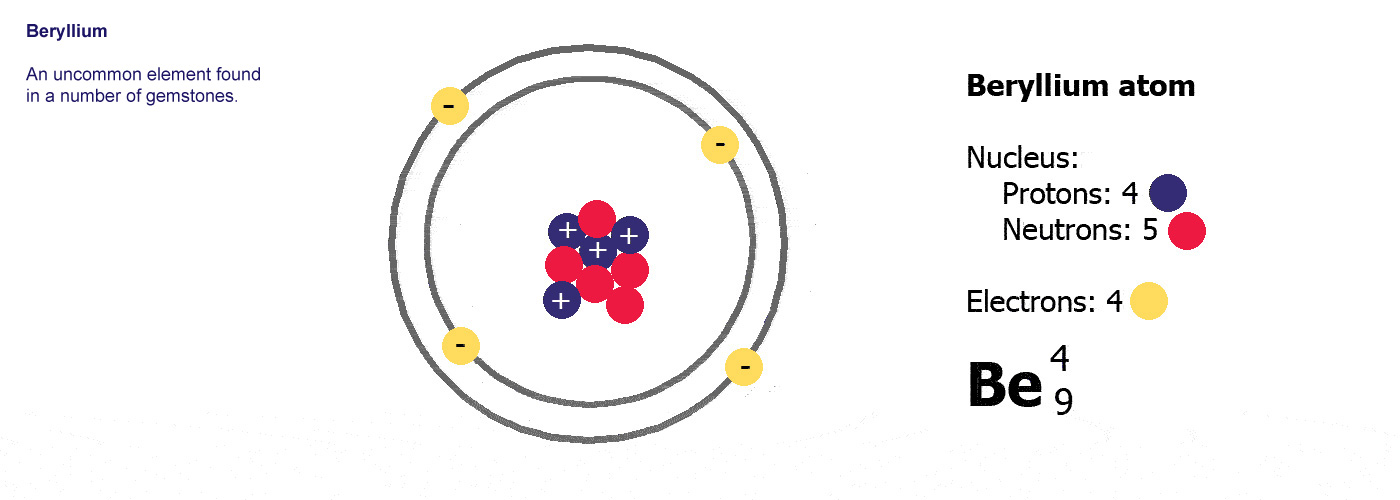
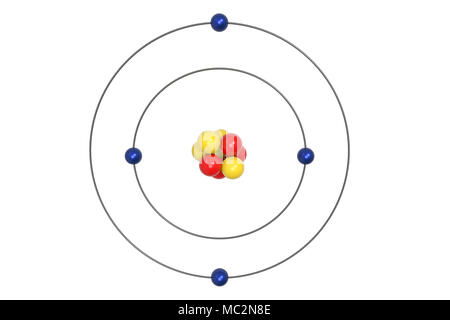
Beryllium – P. E. N 2. A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. What is the pattern for the first 4 she…. Shows how many electrons each shell has. Niels Bohr. The number of protons and neutrons are shown in the centre and…. 2, 8, 8, 18. Bohr Diagram. Shows how many electrons each shell has. Named after. Niels Bohr. Name: Beryllium Symbol: Be Atomic Number: 4 Atomic Mass: 9.012182 amu Melting Point: 1278.0 °C (1551.15 K, 2332.4 °F) Boiling Point: 2970.0 °C (3243.15 K, 5378.0 °F) Number of Protons/Electrons: 4 Number of Neutrons: 5 Classification: Alkaline Earth Crystal Structure: Hexagonal Density @ 293 K: 1.8477 g/cm 3 Color: gray Atomic Structure In the neutral beryllium (Be), how are two 2S electrons moving ? The neutral beryllium has four electrons, in which two 1S electrons are very close to the ...
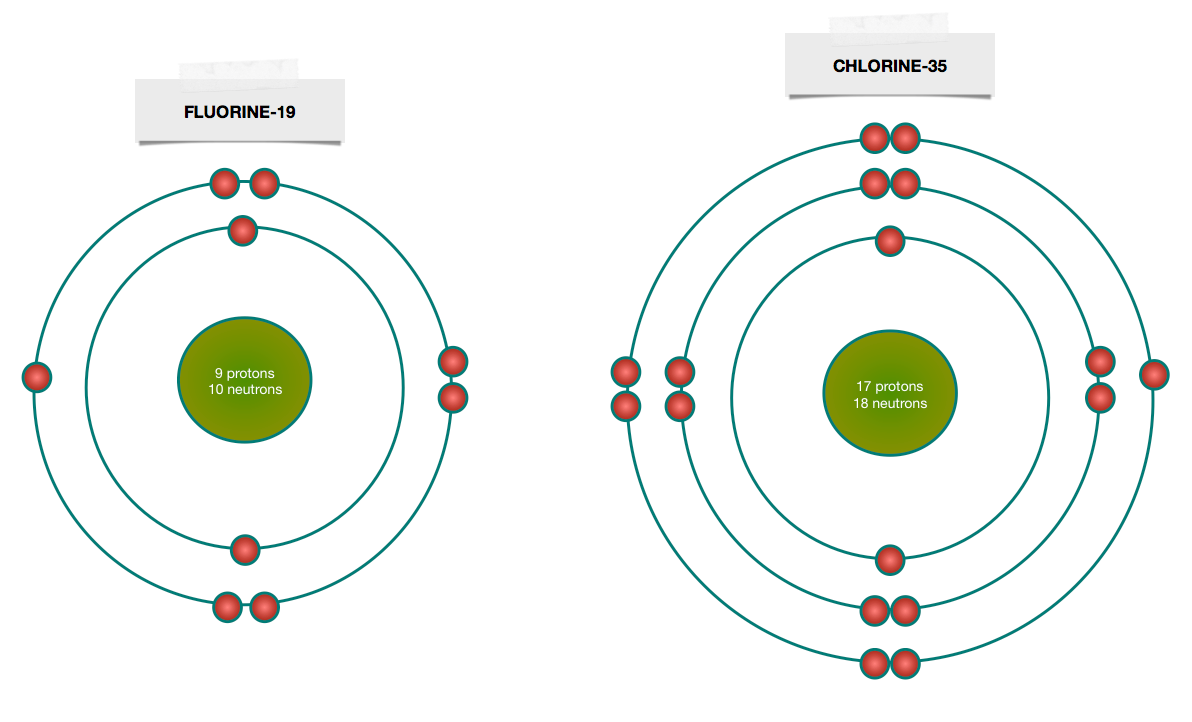
Bohr Model Diagrams and Lewis Dot Structures. Use the information provided for each element to draw Bohr Model diagrams. Rather than drawing individual protons and neutrons, you may simply label how many of each there are in the nucleus (e.g. He: 2p, 2n). Write the element symbol inside of the nucleus. 4. Add energy levels (shells) and electrons for each element. p=2/422. Neon. Lithium beryllium. Boron carbon.8 pages According to Bohr's model of the atom, electrons orbit about the nucleus much like the way planets orbit the sun. Different energy levels are associated with the different orbits. The diagram below shows the Bohr model for fluorine. The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 electrons. 1 Answer BRIAN M. Dec 10, 2014 The Bohr Model for Beryllium (Be) has 4 protons in the nucleus due to the atomic number of Be being 4. The Mass number is 9 which means Beryllium needs 5 neutrons in the nucleus. (Mass number = protons + neutrons, 9 = 4 + n). Beryllium has four electrons to balance the four protons.
Then, draw the individual electrons on the appropriate energy levels (keep in mind the maximum number of electrons allowed on each level). 1. Beryllium ...2 pages
Beryllium difluoride is the fluoride salt of beryllium (+2 oxidation state). In the solid state it exists as a glass, with four-coordinate Be(2+) tetrahedral centres and two-coordinate fluoride centres. As a gas it adopts a linear triatomic structure and in the liquid state a fluctuating tetrahedral structure.
Jan 18, · aluminum, al bohr diagram. Draw a bohr model of this atom the atom has 17 protons, 18 electrons and 20 neutrons. From the periodic table, find the element, and identify its atomic number and atomic. Bohr diagrams 1) add the electrons. Rutherford's model introduced the nuclear model of an atom, in which he explained that a nucleus ….
Bohr Diagram For Beryllium Summarized by PlexPage. Last Updated: 28 October 2020 * If you want to update the article please login/register. General | Latest Info. Beryllium, or Be, is atomic number 4 on the periodic table of elements. This means the beryllium atom has four protons and four electrons. The number of neutrons present varies in beryllium atom, making three isotopes-atoms with ...
Bohr model of all Elements is mentioned in the chart below. ... Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5 : 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr ...
Learn bohr diagrams with free interactive flashcards. Choose from 221 different sets of bohr diagrams flashcards on Quizlet.
The Bohr Model of Beryllium(Be) has a nucleus that contains 5 neutrons and 4 protons. This nucleus is surrounded by two-electron shells named K-shell and ...Total valence electrons in Beryllium: 2Electron in the First shell(K): 2Electrons in the Second shell(L): 2Total electron shells: 2Steps to draw the Bohr Model... · Find Valence electron of...
Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons
In this video we'll use the Periodic table and a few simple rules to find the number of protons and electrons for the Beryllium ion (Be2+). From the Periodic...
diagram wiring diagram database bohr, beryllium bohr diagram drawing, beryllium chloride wikipedia, electron arrangement of the first 20 elements pass my exams, the structure of an atom explained with a labeled diagram, atom diagram universe today, how to represent electrons in an energy level diagram, beryllium atom structure grand unified theory,
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n.
Bohr's atomic model hydrogen emission spectra. Bohr explained that electrons can be moved into different orbits with the addition of energy. When the energy is removed, the electrons return back to their ground state, emitting a corresponding amount of energy—a quantum of light, or photon. What is beryllium Bohr diagram?
Stock market chart value. Made with analog vintage lens, Leica APO Macro Elmarit-R 2.8 100mm (Year: 1993)
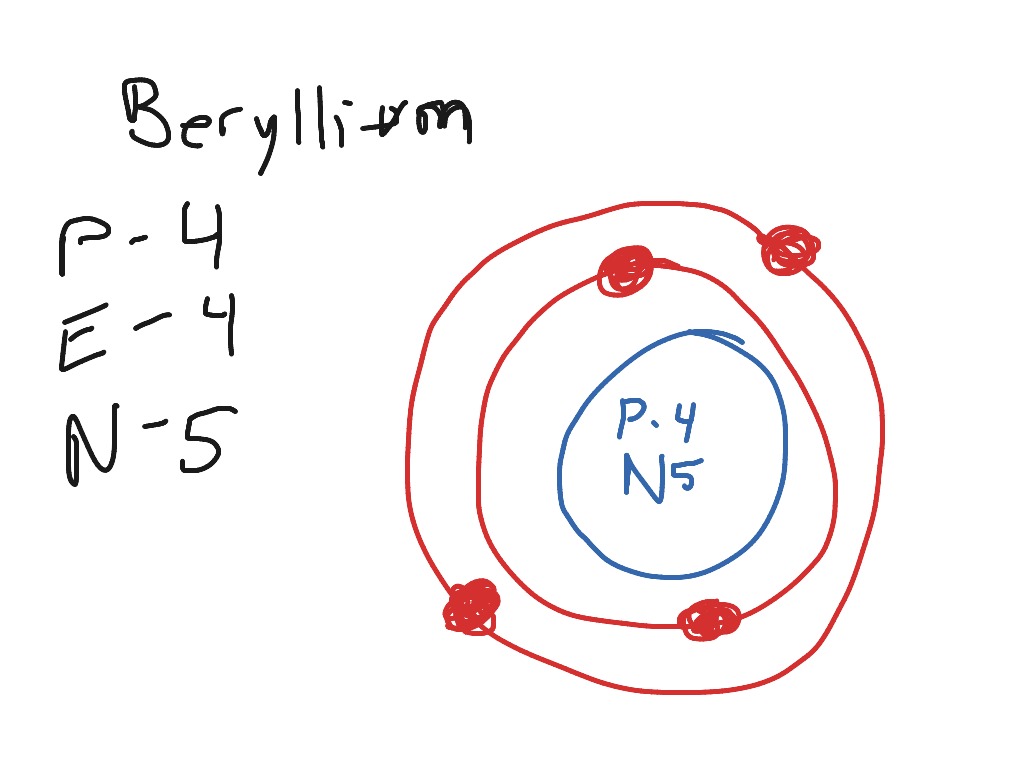
Bohr Model Diagrams. 1. Beryllium -. P- 4 protons. E- 4 electrons. N- 5 neutrons. 2. Sodium -. P- 11 protons. Draw a Bohr Model of Beryllium Draw a Bohr Model of Chlorine Activity E- 11 electrons. N- 12 neutrons.Name Period Date Bohr Model Diagrams. Use the information provided for each element to draw Bohr Model diagrams.
Beryllium Bohr Model Diagram Feb 19, Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are. Apr 24, The isotope beryllium-9, with five neutrons, is the stable form of the atom. Creating a 3D model provides a child with a visual representation of a.
Beryllium 2. Hydrogen 009 5. Sodium 1 8. Magnesium 11. Silicon . Science 9 Chapter 2 — Elements and the Periodic Table Identify the elements whose Bohr model diagrams are shown below. Write the names of the elements in the spaces provided. O O (f) (a) oo (c) O (d) (b) (c) (d) Name Use with textbook pages 64-67.
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He
Beryllium Bohr model by Anthony Ragazzi - October 19, 2012.







0 Response to "39 bohr diagram for beryllium"
Post a Comment