39 bromine lewis dot diagram
SiBr4 Lewis Structure, Geometry, Hybridization, and ... The Lewis dot structure for Bromine is shown. Total valence electrons= 1*(valence shell electron in Si) +4*(valence shell electron in Br )= (1*4) +(4*7)= 4+28= 32. Step 2. Select the central atom. Si atom is chosen as the central atom to provide stability to the molecule and facilitate a better spread of electron density. How to draw BrF5 Lewis Structure? - Science Education and ... It is represented by dots in the BrF5 Lewis diagram. The BrF5 molecule's core bromine atom can be represented as follows: Total outermost valence shell electron of bromine atom in BrF5= 7 Total outermost valence shell electron of fluorine atom in BrF5= 7 The BrF5 molecule has one central bromine and five fluorine atoms.
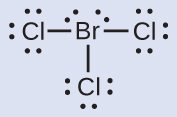
Lewis Structure Hbr - aunitedkingdomfilm.com Bromous acid is one such compound of bromine in which the bromine a central atom has expanded octet. Draw the Lewis Dot Structure for the. Lewis structure is a way of showing the bonding between the constituent atoms by considering the outer shell electrons of each atom. Learn this topic by watching Lewis Dot Structures.

Bromine lewis dot diagram
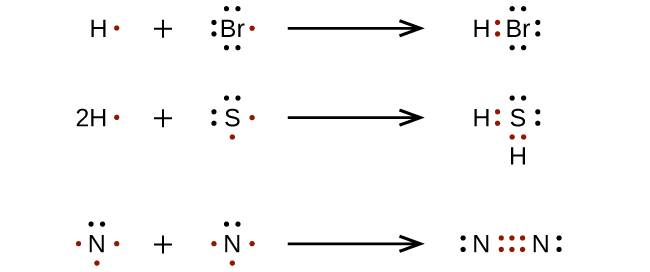
Br2 Lewis Structure: How to Draw the Dot Structure for ... Looking on the periodic table, Bromine is in Group 7 or 17. It has 7 valence electrons. We have two of them though. Multiply that by 2, for a total of 14 valence electrons for the Br2 Lewis structure. First, we'll draw two Bromine atoms next to each other. We have 14 valence electrons for Br2. We'll put two between atoms to form a chemical bond. PDF Aluminum and bromine lewis dot structure Aluminum and bromine lewis dot structure Describe what happens during a phase change. Calculate the changing energy required for a phase change. Substeadies can change the phase - often because of a temperature change. At low temperatures, most substances are solid; As temperature increases, they become liquid; At higher temperatures still ... Lewis dot diagram for bromine? - Answers Jun 14, 2011 · All the halogens atoms, Fluorine , chlorine ,iodine and Astatine have the same dot diagram as the bromine. What is the Lewis dot diagram for Ne neon? the Lewis dot diagram for neon is as follows. .:
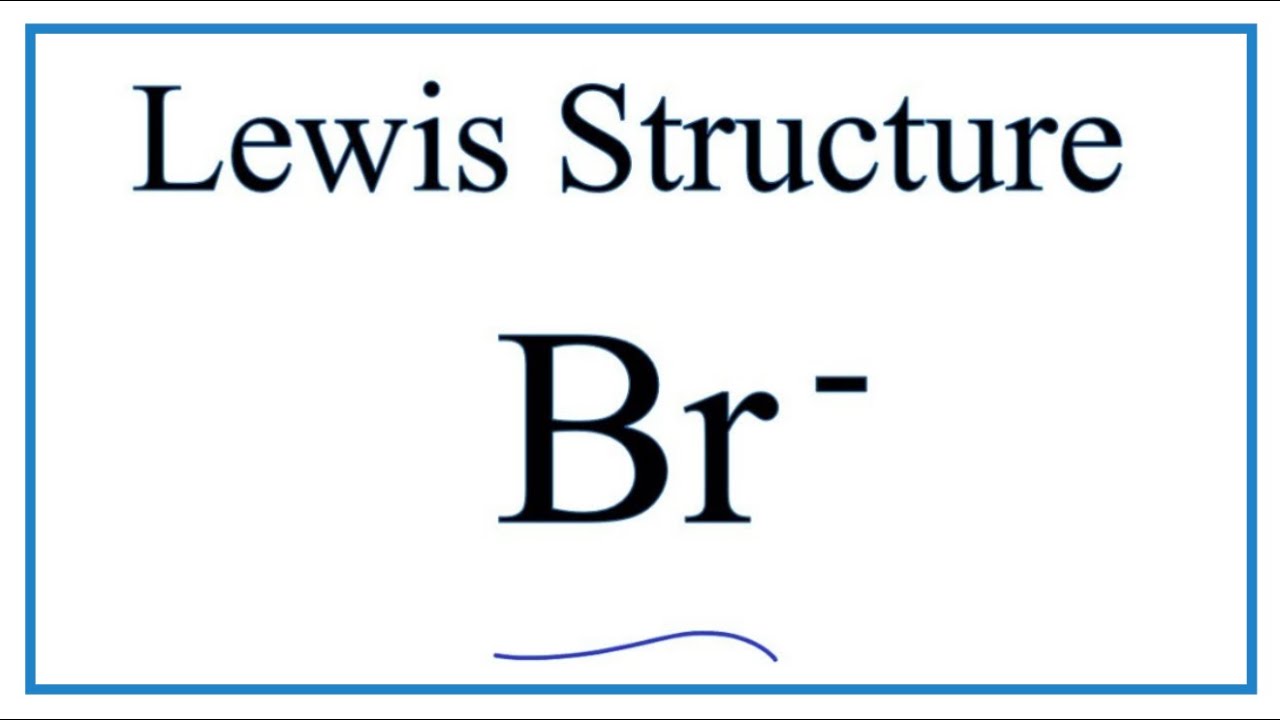
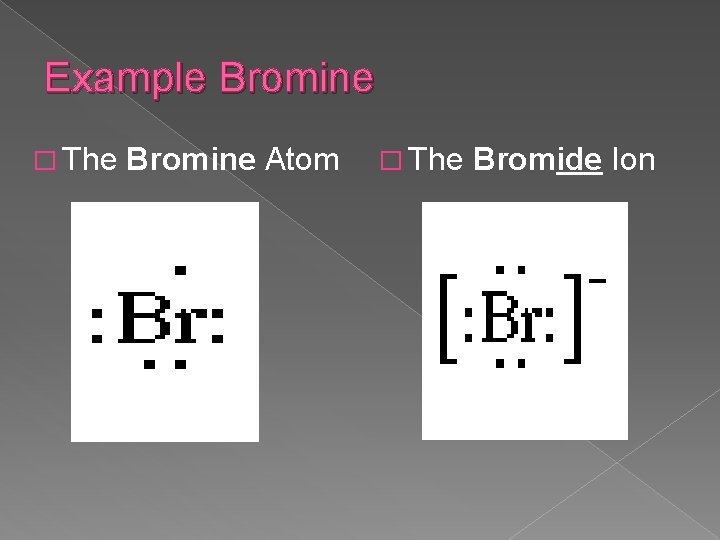
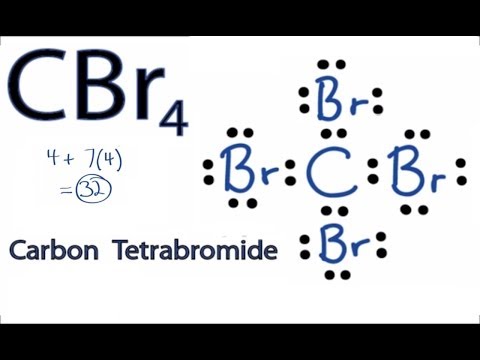
Bromine lewis dot diagram. Lewis Dot Diagrams (Structures) for Atoms and Ions ... Hydrogen / Helium (watch out!) / Bromine Selenium / Nitrogen / Barium Chlorine / Gallium / Argon. WKS 6.2 - LDS for Ions/ Typical Charges. Determine the common oxidation number (charge) for each of the following ions, and then draw their Lewis Dot Structure. Don't forget to show brackets and charge on your LDS for ions! Lewis Structures: Learn How to Draw Lewis Structures ... A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion. Using the Periodic Table to Draw Lewis Dot Structures. The periodic table has all of the information needed to draw a Lewis dot structure. Each Group, or column, is indicated by a roman numeral which represents the number of valence electrons. CBr4 Lewis Structure - How to Draw the Dot Structure for ... The Lewis structure for CBr 4 is similar to CCl 4. Since they are in the same Group on the periodic table they each have the same number of electrons (7) their structures are similar. The Carbon atom goes in the center of the Lewis structure since it is the least electronegative. calcium and bromine lewis structure - srpirrigation.gos.pk Lewis dot diagram for bromine. A oxygen 2 ion b sulfur 2 ion c antimony d aluminum. the physical properties of the molecule (like boiling point, surface tension, etc. M. metcashflow. 4.
How to draw BeBr2 Lewis Structure? - Science Education and ... It is represented by dots in the BeBr2 Lewis diagram. The BeBr2 molecule's core carbon atom can be represented as follows: Total outermost valence shell electron of beryllium atom in BeBr2= 2 Total outermost valence shell electron of atom in BeBr2= 7 The BeBr2 molecule has one central beryllium atom and two bromine atoms. Br2 Lewis Structure - How to Draw the Lewis Dot Structure ... Br2 is also called Bromine gas. ----- Steps to Write Lewis Structure for compounds like Br2 ----- 1. Find the total valence electrons for the Br2 molecule. 2. Put the least electronegative atom in... How to Draw the Lewis Dot Structure for Br ( the Element ... A step-by-step explanation of how to draw the Br Lewis Dot Structure.For the Br structure use the periodic table to find the total number of valence electron... Lewis Dot Structure For Hbr - aunitedkingdomfilm.com The Lewis Dot Structure is a visual which represents the outermost shell of electrons also known as valence electrons and possible covalent bonds within an atom or molecule. Below are the steps to draw the lewis diagram of the Br2 molecule. These valence electrons are negatively charged and are attracted to the positively charged nucleus made up.
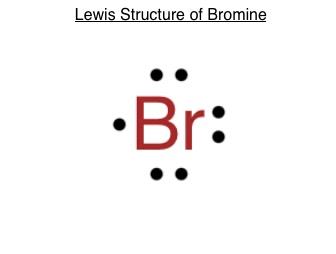
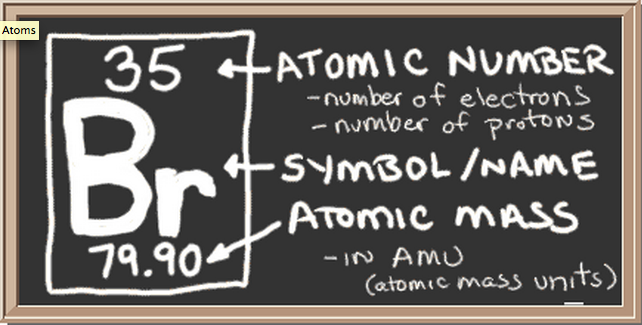
Bromine pentafluoride (BrF5) lewis dot structure ... Follow some steps for drawing the lewis dot structure of BrF5 1. Count total valence electron in BrF5 In the very first step, we need to determine how many valence electrons are available for BrF5. A valence electron is the outermost shell electron associated with an atom. It is represented as dots in the lewis diagram. Bromine - Home Bromine is a nonmetal. Bromine Electron Dot Diagram Since Bromine is in group, or series, 17, it has 7 valence electrons. Group 1 on the periodic table has 1 valence electron. Group 2 on the periodic table has 2 valence electrons. The amount of valence electrons in groups 3-12 cannot be predicted because their oxidation numbers are unknown. How would you represent potassium and bromine using an ... This means that its electron dot diagram will feature its chemical symbol and one dot, usually placed above the symbol. Bromine, "Br", is located in group 17, period 4 of the periodic table. Its electron configuration looks like this ["Br"]: 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 4p^5 Bromine has seven valence electrons, all located on the fourth energy ... BrF5 Lewis Structure, Molecular Geometry, Hybridization ... From the Lewis dot structure of BrF5, it is clear that the electron geometry of the molecule is octahedral where the electronegativity values of bromine and fluorine are 2.96 and 3.98. The difference between both the values is 1.02 which is greater than 0.4 so the BrF5 molecule is a polar molecule.
Bromine | Br2 - PubChem Bromine is an active ingredient in four products; two products with multiple active ingredients and two products as the sole active ingredient. The multiple active ingredient products control mold, mildew, fungi, insects, and odors in exposed surfaces of bedding, mattresses, textiles, drapes, upholstered furniture, rugs, carpets, and storage areas.
Bromine (Br2) Lewis Structure - chemistryscl.com Lewis structure of bromine contains only one Br-Br bond and each bromine atom has three lone pairs. It is very easy to Br 2 lewis structure. Br 2 lewis structure. There is a single bond with bromine atoms and three lone pairs on each bromine atoms. So, this lewis structure is a very simple. Steps of drawing lewis structure of Br 2
Bromine Lewis Dot Structure: Drawing, Several Compounds and ... Bromine Lewis dot structure represents that bromine is a diatomic molecule with the formula Br 2. This article explains the bromine Lewis dot structure with itself and other elements along with its visual representation. Bromine belongs to group 17 of the periodic table. So there is a total of 7 electrons in its valence shell.
BrO3- lewis structure, molecular geometry, bond angle ... Follow some steps for drawing the lewis dot structure for BrO3- 1. Count total valence electron in BrO3- Lewis diagram is a simple representation of valence electron within a molecule. So, for determining the valence electron in BrO3-, look at the periodic group of bromine and oxygen atoms.
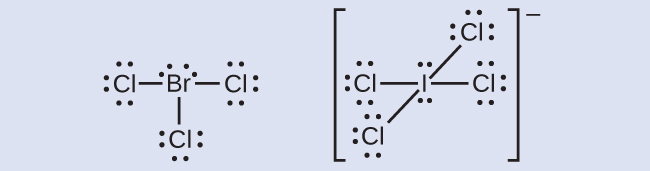
Brf3 Lewis Structure: Draw the Bromine Trifluoride Dot ... So to understand the Lewis Dot structure of BrF3, let's first know some basic details needed to make this structure. BrF3 Valence Electrons Bromine has seven electrons in its valence shell, and fluorine also has seven electrons in its outer shell. To get the total number of valence electrons, we have to add all these electrons: Br = 7 electrons
SBr6 Lewis Structure: Drawings, Hybridization, Shape ... sbr6 lewis structure: SBr6 or sulfur bromide is an inorganic compound which form by covalent bonding. So the bonding in sulfur bromide can be explained by the concept of lewis dot structure. So we will study SBr6 lewis structure in detail and various facts related to it in the following sections.
Lewis dot diagram for bromine? - Answers Jun 14, 2011 · All the halogens atoms, Fluorine , chlorine ,iodine and Astatine have the same dot diagram as the bromine. What is the Lewis dot diagram for Ne neon? the Lewis dot diagram for neon is as follows. .:
PDF Aluminum and bromine lewis dot structure Aluminum and bromine lewis dot structure Describe what happens during a phase change. Calculate the changing energy required for a phase change. Substeadies can change the phase - often because of a temperature change. At low temperatures, most substances are solid; As temperature increases, they become liquid; At higher temperatures still ...
Br2 Lewis Structure: How to Draw the Dot Structure for ... Looking on the periodic table, Bromine is in Group 7 or 17. It has 7 valence electrons. We have two of them though. Multiply that by 2, for a total of 14 valence electrons for the Br2 Lewis structure. First, we'll draw two Bromine atoms next to each other. We have 14 valence electrons for Br2. We'll put two between atoms to form a chemical bond.

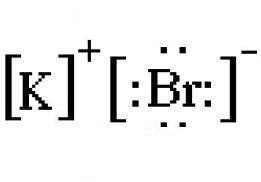





















0 Response to "39 bromine lewis dot diagram"
Post a Comment