42 newman projection energy diagram
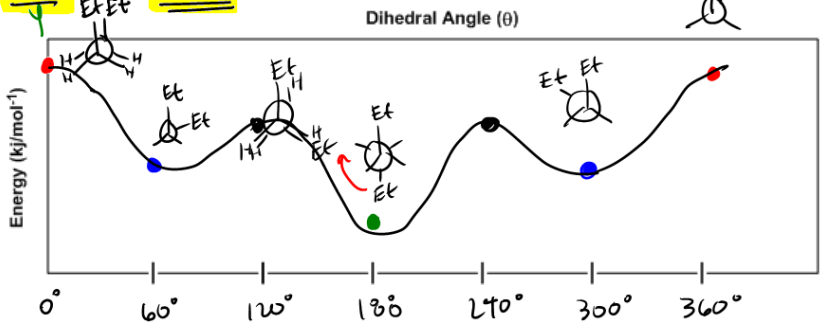
Newman Projections - Organic Chemistry Tutor Newman Projections. When atoms in organic molecules connect via single bonds, you can have a free rotation around those single bonds. That gives you a different 3D forms of the same molecule. We refer to those forms as conformations of the molecules. These molecular conformations may be somewhat challenging to represent. PDF 331 Worksheet Week 4 Draw the Newman projections for ... Draw the Newman projections for pentane looking down the C2-C3 bond through a full 360 degree rotation. Start with the anti-staggered Newman projection then draw and label each ... The large energy difference between the diequatorial and diaxial conformations is due to the 1,3 diaxial interaction between the two methyl groups. 1b. Approximately ...
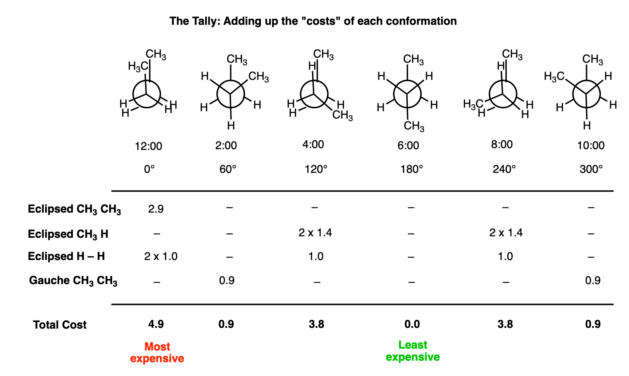
PDF Newman Projections - More Practice- Answer Key III. For each of the following, determine what strain energy is involved in each Newman projection (torsional and/or steric) to explain why the first Newman projection is more stable than the second. (-Cl is smaller than any alkyl group) a. Butane, C2-C3 (front carbon is C2) STERICS 3 b. Butane, C2-C3 (front carbon is C2) TORSIONAL
Newman projection energy diagram
Newman Projections - Organic Chemistry Video | Clutch Prep Concept #3: How to draw a Newman Projection Energy Diagram. Expert Q&A. Ask unlimited questions and get expert help right away. Report issue. Transcript. So, what I want to do is I want to plot an energy diagram with these degrees and I want to show you guys what that means and how that actually translates, OK? Now I know this is going to be ... Newman Projections - Video Tutorials by Leah4sci Newman projections are important in organic chemistry to help you understand one aspect of molecular conformations. These videos will help you understand everything from Newman projection basics, how to convert Newman to other forms, how to analyze the energy of particular conformations, and of course how to draw an energy diagram. Drawing Newman Projections - Organic Chemistry Video ... Newman projections are head-on representations of molecules looking down the bonds between two carbons typically used to visualize rotation around a single bond. We refer to these different rotations of Newman Projections as conformations. ... Energy Diagrams. Let's put all of that together and look at a visual representation of the different ...
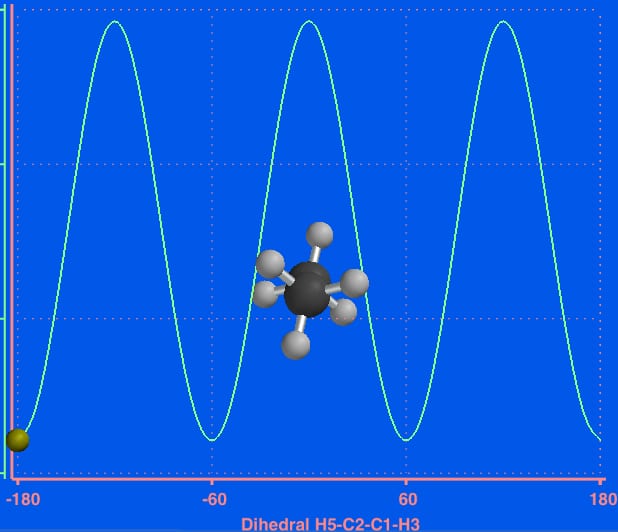
Newman projection energy diagram. More On 1,2 and 1,4 Additions To Dienes – Master Organic ... 2017-04-11 · 1. Recap: “1,2” vs. “1,4” Addition To Dienes, And “Kinetic Control” vs “Thermodynamic Control “ To recap: In Org 1 we learned that addition of HCl and HBr to normal, isolated alkenes (such as 1-butene) just gives one product – the Markvonikoff product (“1,2-addition”) where the H and the nucleophile (e.g. Br-) are on adjacent carbons, and Br has … A Bulky Reducing Agent For Esters - Master Organic Chemistry 2011-08-26 · DIBAL (Di-isobutyl Aluminum Hydride) – A Bulky Reducing Agent For The Partial Reduction Of Esters. In a blatant plug for the Reagent Guide, each Friday I profile a different reagent that is commonly encountered in Org 1/ Org 2.Version 1.2 just got released, with a host of corrections and a new page index. Energy Diagram of n-Butane Rotations - Newman Projection ... This is an energy diagram of the conformations of n-Butane calculated using Spartan from Wavefunction. The 2,3 bond was set for torsional angles starting at ... Newman projections (video) - Khan Academy Now let's draw the eclipsed conformation as a Newman Projection. So as a Newman Projection the front's going to look the same. You have a hydrogen there, you have this hydrogen, you have that hydrogen, and then you have that blue, or I guess that purple hydrogen, down here. So that's the front of it.
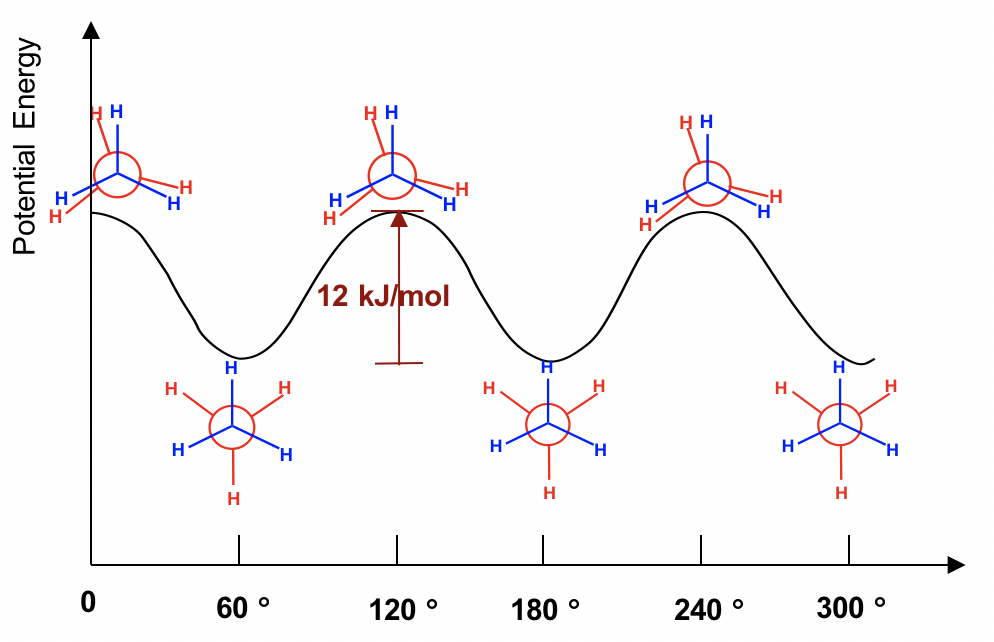
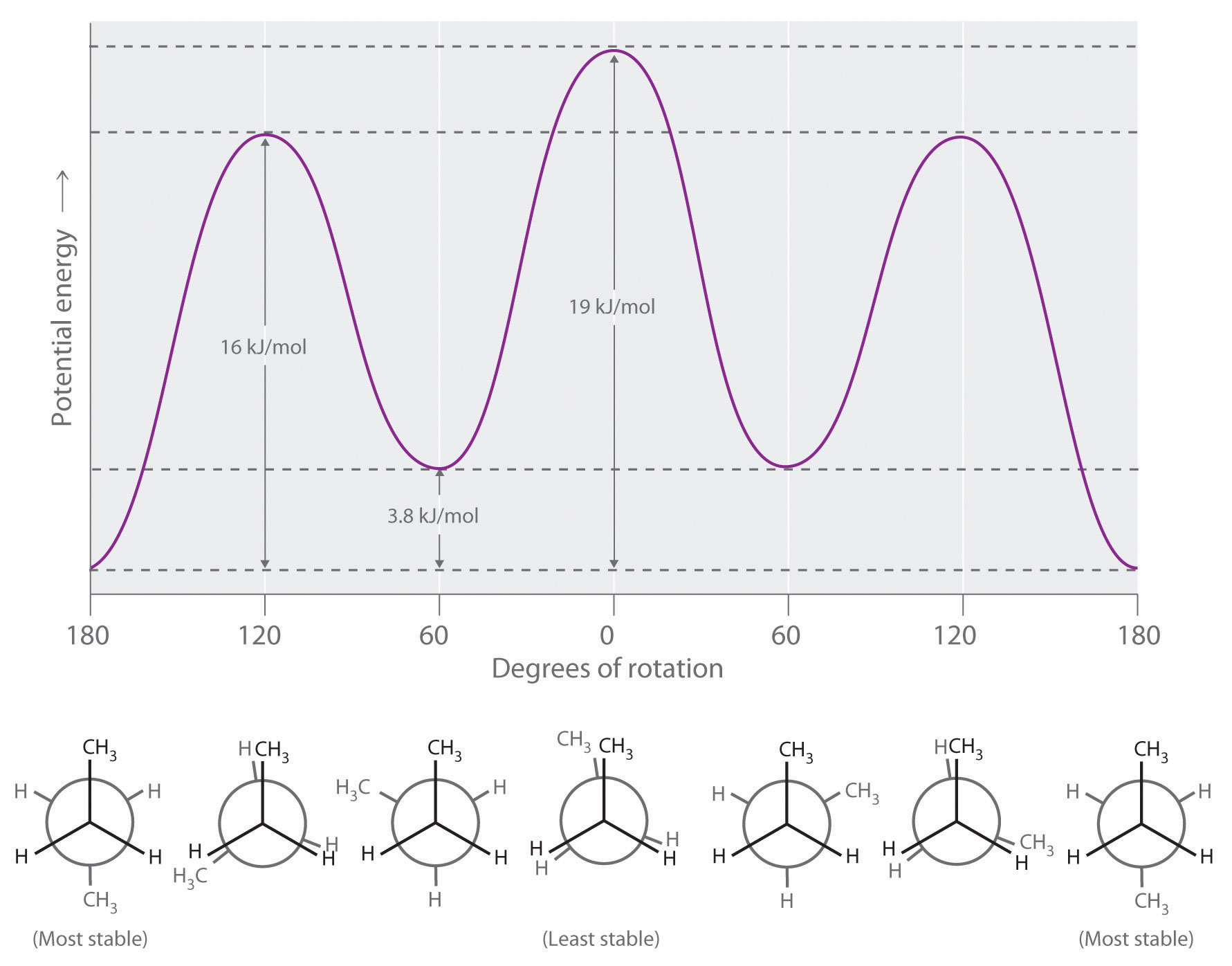
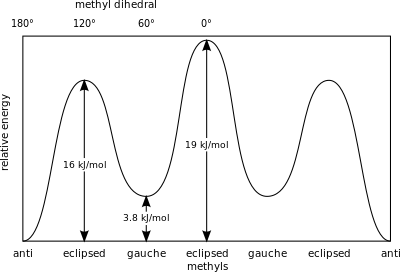
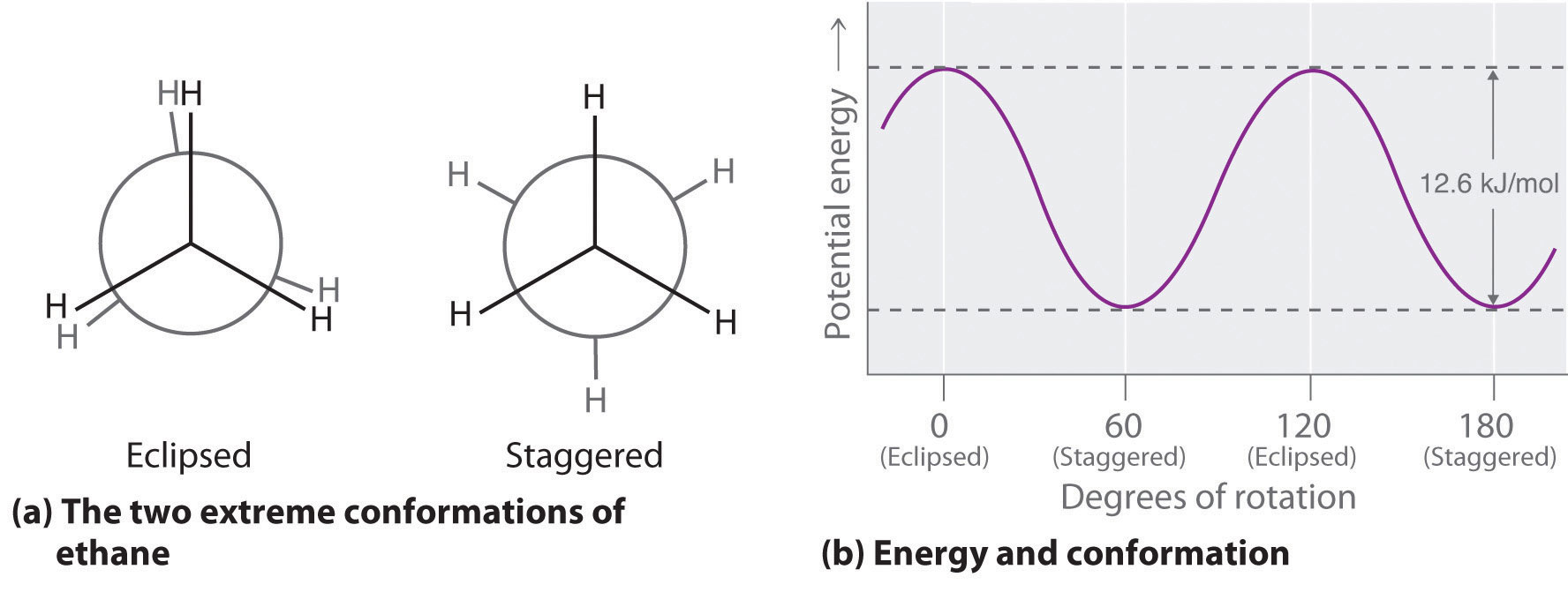
Newman projection practice problems [with a free book of ... Draw the energy diagram for a Newman projection: Energy diagrams show the relative energy of a molecule compared to rotation about the axis of interest. Generally, these start at 0 degrees and rotate through the entire molecule. This can then be graphed showing which parts and bond angles about the axis of interest are more or less stable. Newman Projections and Practice on Newman Projections A Newman projection is a representation of the molecule looking through a C-C single bond. For every Newman projection, you need to specify the bond and the direction you are looking at. For example, for our molecule, we can look through the C1-C2 bond (even though it can be through any bond). The direction is usually shown with an eye symbol: Conformations of ethane - Newman projection Conformations of ethane - Newman projection. The two conformational energy diagrams shown below are staggered ethane and eclipsed ethane. The relative energies of each conformer are shown. The staggered conformer is lower in energy than the eclipsed conformer. Click on the energy diagrams to see a movie of the conformers with their relative ... Instability of the Kerr Cauchy horizon under linearised ... 2022-01-31 · Standard energy estimates entail that solutions of linear equations arising from regular initial Cauchy data can at most become singular at the (null) boundary of the black hole interior, i.e., at the Cauchy horizon
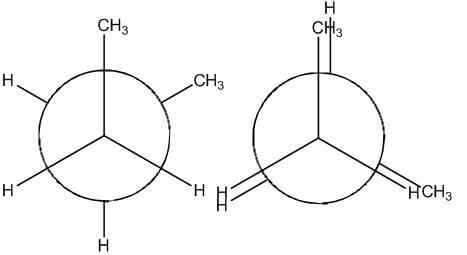
Conformers - Definition, Newman Projection, Conformation ... The following diagram shows the full rotation about a C-C single bond, the relative energy contents, and the names of the conformers depending on the dihedral angle. Eclipsed groups with bonds enclosing dihedral angles of 0o in the Newmann projection suffer from overcrowding in space, in this situation they develop the most intense steric ... Introduction to Newman Projections - Organic Chemistry ... Explanation: Groups connected to both the front and back carbons are drawn using sticks at 120° angles. A sawhorse projection is similar to a Newman projection, but it shows the carbon-carbon bond that is hidden in a Newman projection. Just as with Newman projections, you can draw sawhorse projections in eclipsed and staggered conformations ... 8 Newman Projection Formulas and Energy Diagrams ... About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... 3.4.1. Newman Projections - Chemistry LibreTexts The figure below shows, as an example, a Newman projection looking down the C 2-C 3 bond of octane. Exercise 3.1: Draw Newman projections of the lowest and highest energy conformations of propane. Exercise 3.2: Draw a Newman projection, looking down the C 2 -C 3 bond, of 1-butene in the conformation shown below (C 2 should be your front carbon).
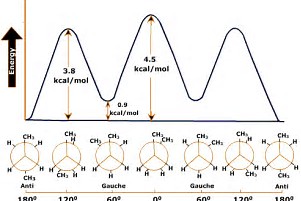
Conformational Analysis of Butane Using Newman Projections ... Professor Davis demonstrates the conformational energy diagram of butane using Newman projections. Anti, syn, guache and staggered conformations are all dem...
Newman Projection Potential Energy Diagram (Conformational ... This video explains how we perform a conformational analysis on a given molecule and draw its potential energy diagram. A conformational analysis typically c...
Drawing Newman Projections + Staggered & Eclipsed ... The overlap, and the energy difference associated with this overlap, leads to two energetic subgroups of Newman projections: eclipsed and staggered. Eclipsed conformations result in more steric hindrance between two atoms than staggered conformations because of how close the atoms can get to one another. Eclipsed conformations are therefore ...
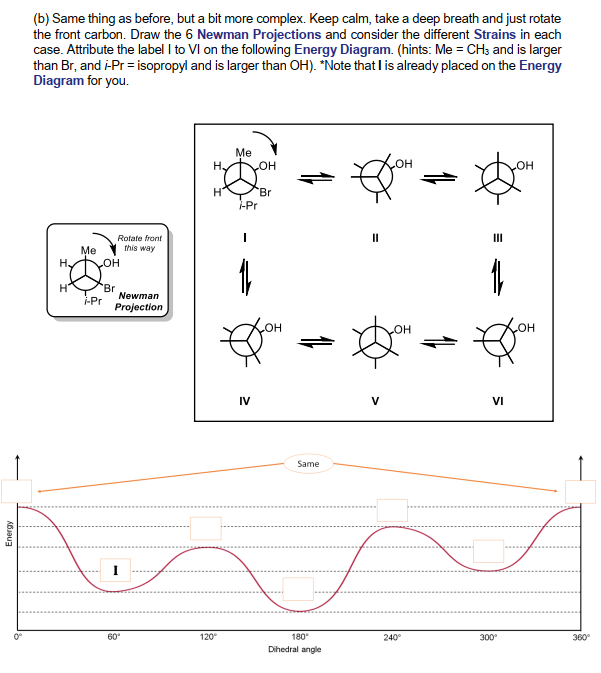
PDF Cyclohexane Chair Analysis Newman Projections and Practic ... Draw a qualitative energy diagram for CH 3CH 2CH 2CH(CH 3) 2, relative to the bond between the two CH2 carbons. The Newman projections are drawn below, using "iPr" as an abbreviation for the isopropyl CH(CH 3) 2group. Put "S" (for staggered) by any "staggered" conformation, and "E" (for eclipsed) by an eclipsed conformation. H iPr H CH3
Quantum gravity - Wikipedia Quantum gravity (QG) is a field of theoretical physics that seeks to describe gravity according to the principles of quantum mechanics, and where quantum effects cannot be ignored, such as in the vicinity of black holes or similar compact astrophysical objects, and where the effects of gravity are strong, such as neutron stars.. Three of the four fundamental forces of physics are …
Newman Projection Energy Diagrams in Organic Chemistry The Newman Projection is an important skill for every organic chemistry student. Not only does it provide you with another perspective of organic compounds, but it also gives you a means to analyze the conformational energy of a molecule. In this article I will give you a quick introduction to the energy diagram of a Newman Projection.
Drawing Newman Projections - Organic Chemistry Video ... Newman projections are head-on representations of molecules looking down the bonds between two carbons typically used to visualize rotation around a single bond. We refer to these different rotations of Newman Projections as conformations. ... Energy Diagrams. Let's put all of that together and look at a visual representation of the different ...
Newman Projections - Video Tutorials by Leah4sci Newman projections are important in organic chemistry to help you understand one aspect of molecular conformations. These videos will help you understand everything from Newman projection basics, how to convert Newman to other forms, how to analyze the energy of particular conformations, and of course how to draw an energy diagram.
Newman Projections - Organic Chemistry Video | Clutch Prep Concept #3: How to draw a Newman Projection Energy Diagram. Expert Q&A. Ask unlimited questions and get expert help right away. Report issue. Transcript. So, what I want to do is I want to plot an energy diagram with these degrees and I want to show you guys what that means and how that actually translates, OK? Now I know this is going to be ...


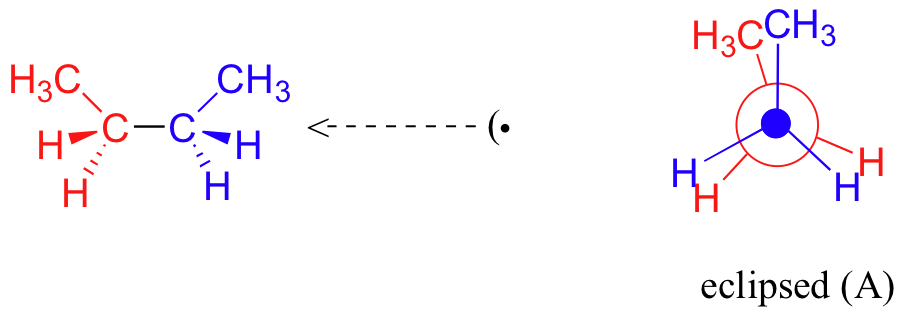



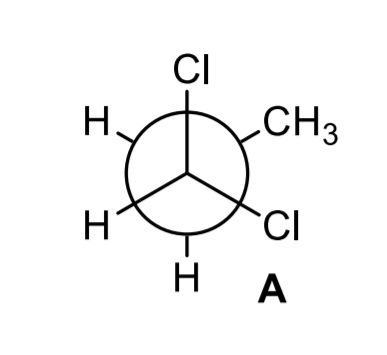
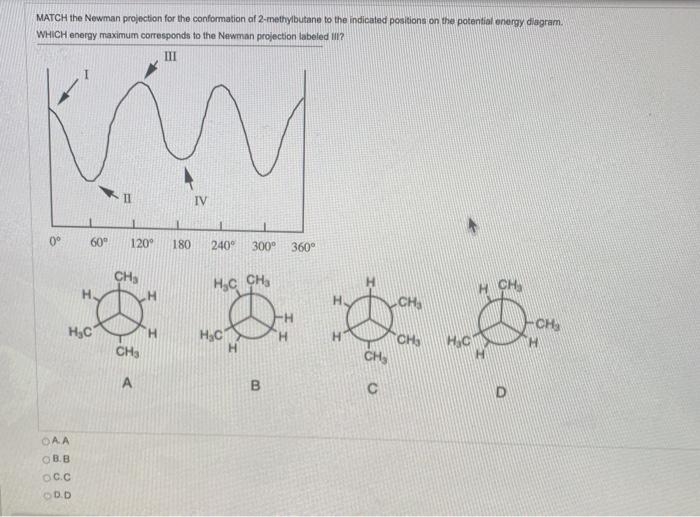

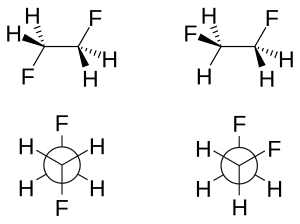



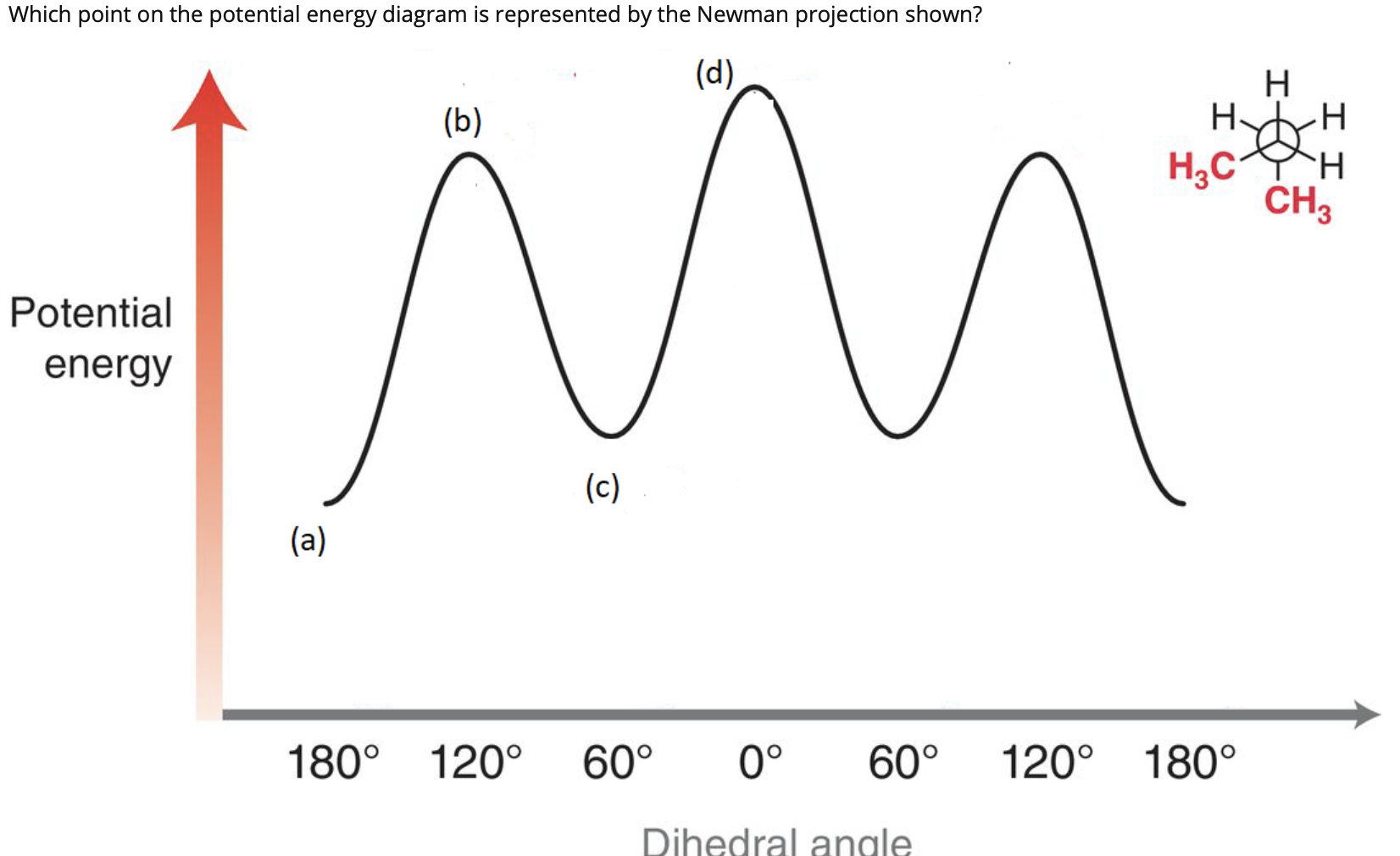








![Solved] Draw a graph, similar to Figure 3-9, of the torsional ...](https://s3.amazonaws.com/si.question.images/images/question_images/1593/4/1/2/2285ef98a841b2011593412225642.jpg)
0 Response to "42 newman projection energy diagram"
Post a Comment