38 molecular orbital diagram for ne2 2+
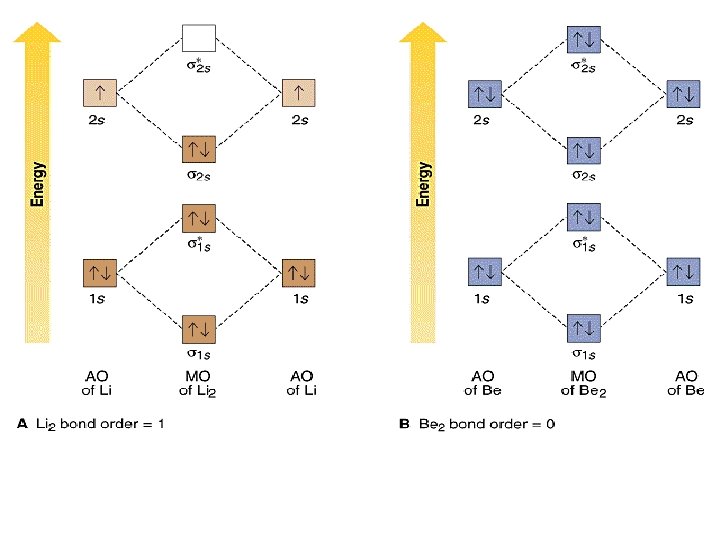
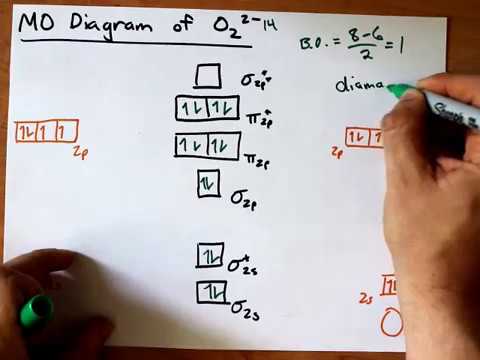
The molecular orbital electronic configuration, Magnetic property: Since bond order is zero, Be 2 molecule does not exist. It is diamagnetic due to the absence of any unpaired electron. B 2 molecule: The electronic configuration of B atom (Z = 5) is. B 2 molecule is formed by the overlap of atomic orbitals of both boron atoms. A number of ... Answer: Yes O2 (2+) is diamagnetic. Explanation: We can work this out by looking at the molecular orbital diagram of O2 O2 (2+) has two fewer electrons than O2 which is what it gives it positive charge. And show diamagnetic behavior as have no unpaired electron. Molecular orbital diagram of ...
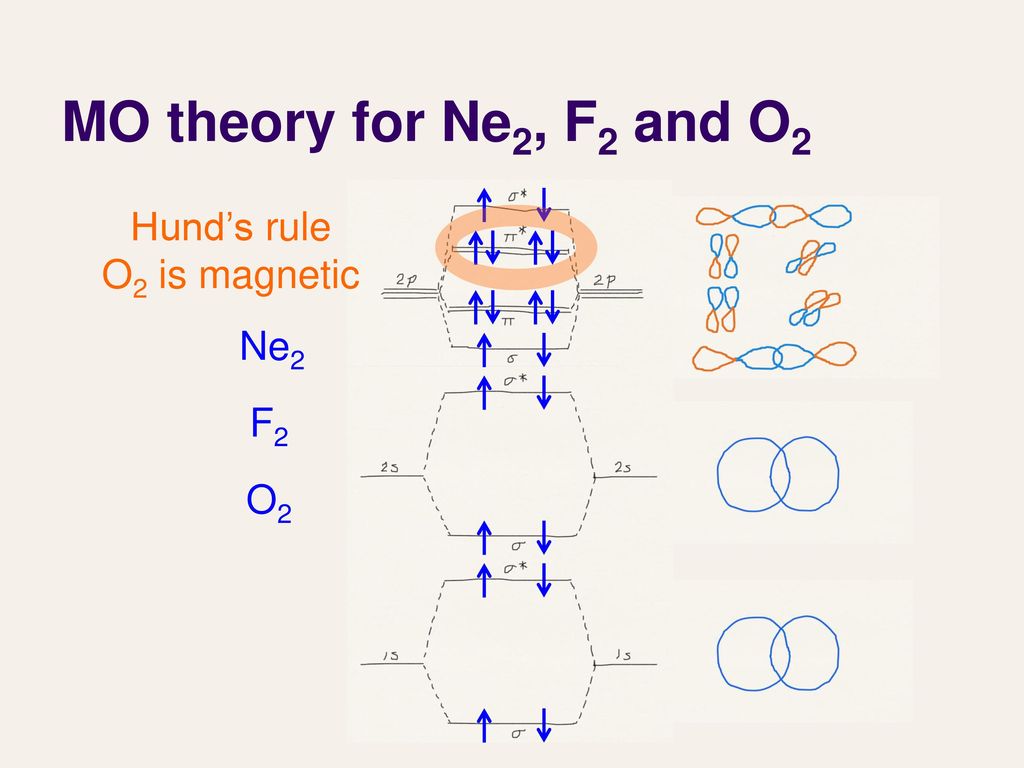
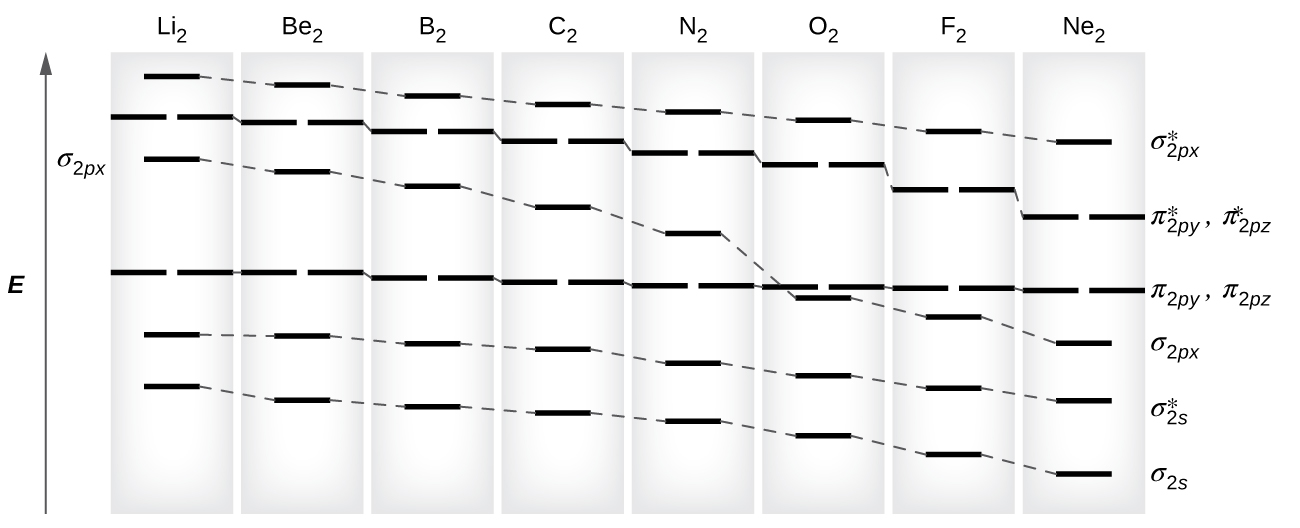
sorry about that not being a molecular orbital diagram i saw orbital and immediately thought electron configuration to save confusion, could. There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc) . One is for the elements up to Nitrogen. The other is for AFTER nitrogen.
Molecular orbital diagram for ne2 2+
This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. . × Close Log In. Log in with Facebook Log in with Google. or. Email. Password. Remember me on this computer. or reset password. Enter the email address you signed up with and we'll email you a reset link. ... A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ... Use a molecular orbital diagram to determine the bond order in the N2+ ion. Write a valence-electron configuration [ (s2s)2 … ] for this ion. (pi lower than sigma) Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.
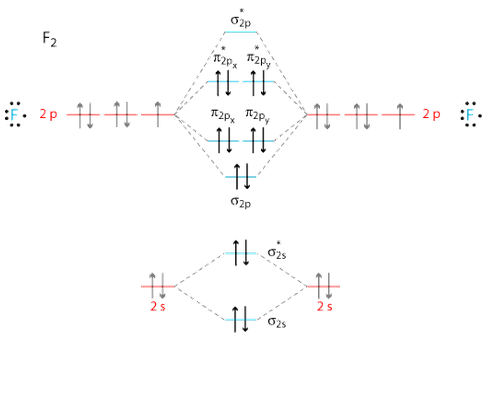
Molecular orbital diagram for ne2 2+. There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start... Biblioteca en línea. Materiales de aprendizaje gratuitos. 1000 Solved Problems in Classical Physics Ahmad A. Kamal 1000 Solved Problems in Classical Physics An Exercise Book 123 Dr. Ahmad A. Kamal Silversprings Lane 425 75094 … Molecular orbital diagram of H 2 (Hydrogen molecule). Molecular Orbital Diagrams of Diatomic Molecules Introduction: In chemistry molecular orbital (MO) theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the ... Answer (1 of 6): Here is the solution, > * For O2 molecule, > * For F2 molecule, Thanks for reading.
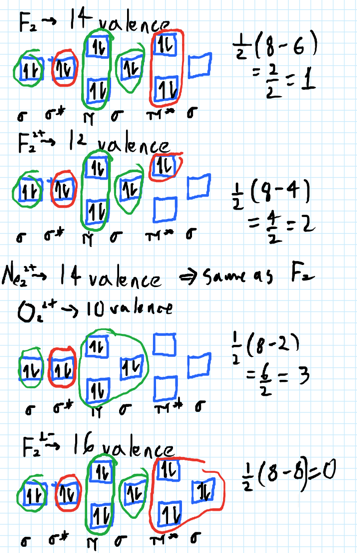
In this video lecture, the MOEL for different Oxygen species lke O2, O2+, O2-, O2 2- , F2 and Ne2 are elaborated. Their electronic configurations, bond order... For Ne 2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 schematron.org each MO an appropriate label. Determine the electron configuration and bond order for each, and rank the three species in order of increasing bond order% (1). From Molecular Orbital Diagram, which is most stable? A. F2 2-B. Ne2 2+ C. O2 2+ D. F2 E. F2 2+ C. O2 2+ Choose the compound below that should have the highest melting point according to the ionic bonding model. A. CaS B. NaCl C. RbI D. MgO E. AlN. E. AlN. A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. According to the molecular orbital theory, in a supposed He2 molecule, both the if we draw its MOT DIAGRAM, 2 e's enter the Bonding molecular Orbital and 2 .
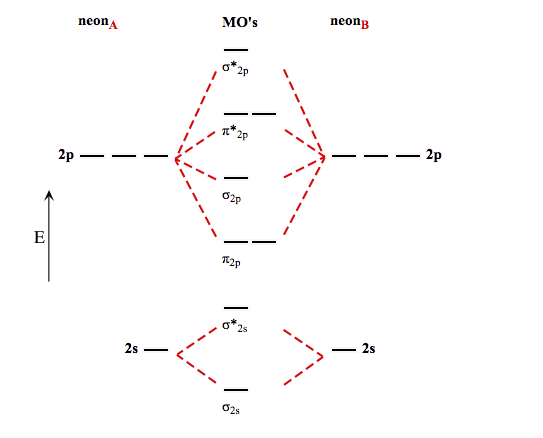
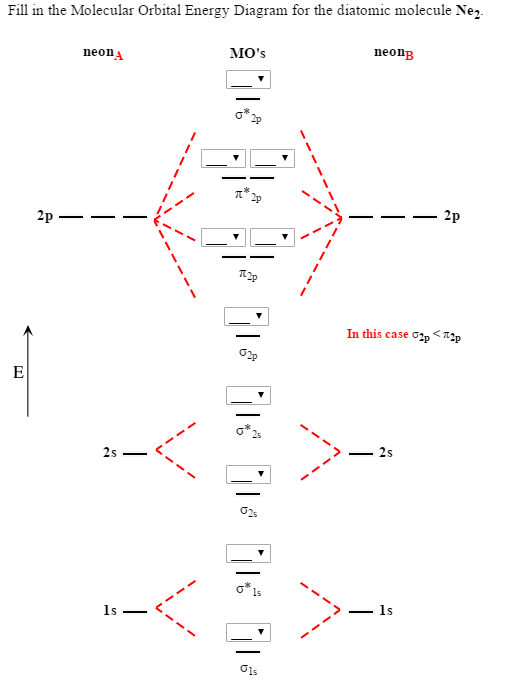
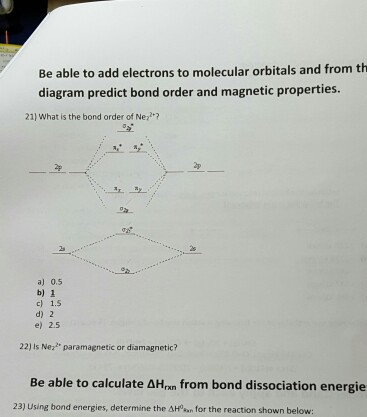
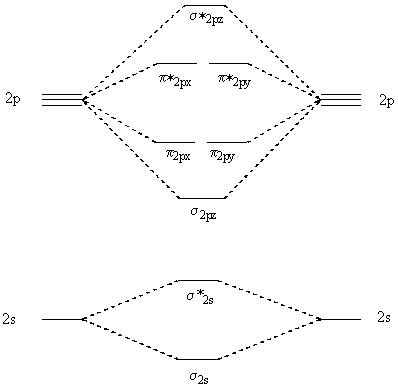
With the help of molecular orbital diagram show that Ne2 cannot exist as stable molecule. (Atomic number of Ne = 10). chemical bonding molecular structure class-11 1 Answer +1 vote answered Jan 26, 2020 by Nishu03 (64.2k points) selected Jan 27, 2020 by SurajKumar Best answer Ne2 (20) = σ1s2 σ*1s2, σ2s2 σ*2s2, σpx2 π 2py2 2π* 2py2π*2py2 2pz2 σ* the pi(2p) bonding orbitals are LOWER than the sigma(2p) bonding orbitals.N2(2-) has a bonding order of 2, which predicts that there will be a stable double ... A) O2^2-B) Ne2^2+ C) O2^2+ D) F2^2+ E) None of the above are paramagnetic; 3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic. A) B2^2+ B) B2^2-C) N2^2+ D) C2^2-E) B2; 4) Draw the molecular orbital diagram shown to determine which of the following is most stable. Draw the molecular orbital (MO) electron diagram for the Ne2 molecule. Be sure your diagram contains all of the electrons in the molecule, including any core electrons. Energy ; Question: O STRUCTURE AND BONDING Drawing the MO energy diagram for a Period 2 homodiat... Draw the molecular orbital (MO) electron diagram for the Ne2 molecule.
Bond order = n bonding electrons − n antibonding electrons 2. Here is the molecular orbital diagram of CN-: There are 8 bonding electrons and 2 antibonding electrons, therefore B. O. = 8 − 2 2 = 3. Here’s a guide on how to construct MO diagrams, in case you need help. 58.1K views · View upvotes · View 1 share · 99 41. 9 1. Related Answer. Jonathan Kurian, Student at The …
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
WESTERN PRODUCTS 7777 NORTH 73RD STREET P.O. BOX 23045 MILWAUKEE, WISCONSIN 53223 -8 " e T 6 M_ I a ... PARTS DIAGRAM & LIST 3' 26 QTY. DESCRIPTION 1 1 1 1 1 1 2 1 2 2 1 1 1 1 1 1 4 16 2 10 2 2 4 6 6 ... and practices when attaching snowplow, including wearing safety glasses during drilling.
Molecular orbital diagram ne2.If ne 2 did form, it would be diamagnetic. Once you have the molecular orbitals and their energy ordering the ground state configuration is found by applying the pauli principle, the what is the molecular orbital diagram for the diatomic neon molecule, ne2?
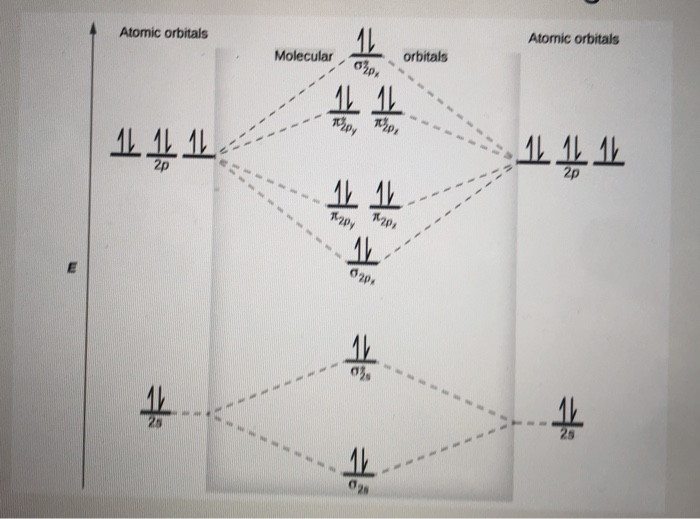
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular…
When two carbons atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals.C2(2-) has a bond order of 3, so i...
Academia.edu is a platform for academics to share research papers.
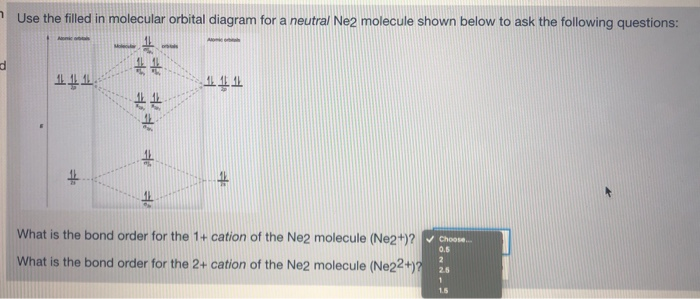
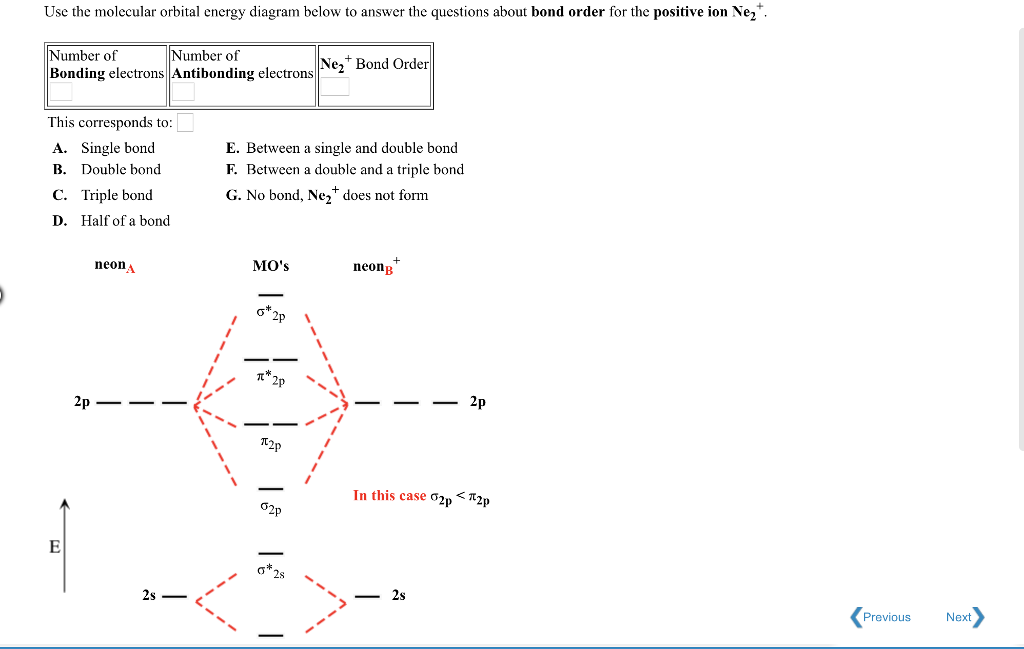
Answer to For Ne2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 anion. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining . Draw the Lewis Structure of Ne2. 1. Draw the atomic and hybrid orbitals on on side of the page.
Use molecular orbital theory to explain why the Be 2 molecule does not exist. Answer: Question 36. Compare the relative stability of the following species and indicate their magnetic properties: O 2, O 2, O 2 – (Superoxide),O 2 2-(peroxide) Answer: O 2 — Bond order = 2, paramagnetic O 2 + — Bond order = 2.5, paramagnetic O 2 – — Bond order = 1.5, paramagnetic O 2 2-— Bond …
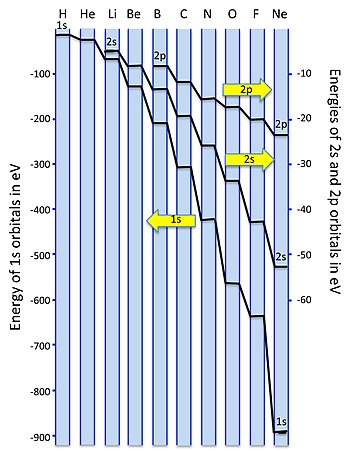
The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
39 molecular orbital diagram for h2o Written By Rosa B. Pruitt Tuesday, January 25, 2022 Add Comment Edit Mercury is the smallest planet in the Solar System and the closest to the Sun.Its orbit around the Sun takes 87.97 Earth days, the shortest of all the Sun's planets.
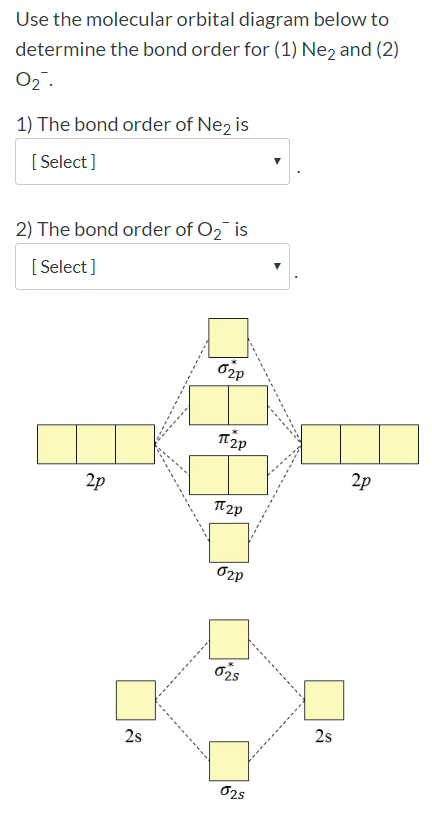
Chemistry. Chemistry questions and answers. Question 2 1 pts Use the molecular orbital diagram below to determine the bond order for (1) Ne2 and (2) 02 1) The bond order of Ne2 is 2) The bond order of O2 is 2p 2p t2p 2p 2s 2s 2s 2s.
Molecular orbital confuguration of Ne2 is σ1s²σ*1s²σ2s²σ*2s²σ2Pz²π2Px²π2Py²π*2Px²π*2Py²σ*2Pz² Hence the bond order of Ne2 according to M.O.T is = (Nb-Na)/2 = (10-10)/2 (since,both bonding orbitals and non-bonding orbital contains 10 electrons) =0 Hence, no bond is possible between 2 Ne atom. Therefore, formation of this molecule is not possible.
The orbital correlation diagram in predicts the same thing--two electrons fill a single bonding molecular orbital. To further demonstrate the consistency of the Lewis structures with M.O. theory, we will formalize a definition of bond order--the number of bonds between atoms in a molecule. For $\ce {N2-}$ there are 15 electrons.
0:21 Molecular Orbital Diagram of Oxygen Molecule3:30 Molecular Orbital Diagram of Florine Molecule5:25 Molecular Orbital Diagram of Neon MoleculeSo as we d...
Nov 21, 2018 · According to Molecular Orbital theory, only those molecule can exists which have net positive bond order while the molecules with negative or. Answer to For Ne2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 anion. © Prof Adam J Bridgeman | close window. © Prof Adam J Bridgeman | close window.The orbital correlation diagram in predicts the same thing--two electrons fill a single bonding molecular orbital.
Use a molecular orbital diagram to determine the bond order in the N2+ ion. Write a valence-electron configuration [ (s2s)2 … ] for this ion. (pi lower than sigma) Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.
A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ...
This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. . × Close Log In. Log in with Facebook Log in with Google. or. Email. Password. Remember me on this computer. or reset password. Enter the email address you signed up with and we'll email you a reset link. ...

![Expert Verified] using MOT find out the bond order of Ne2 ...](https://hi-static.z-dn.net/files/d9f/f4e9ccc0480e0f453254060b5fef8c9a.jpg)
![Expert Answer] Draw the molecular orbital diagram for F2 and ...](https://hi-static.z-dn.net/files/dae/d7baa23a1d4a2ea2c90e0a703e2fd41d.jpg)











0 Response to "38 molecular orbital diagram for ne2 2+"
Post a Comment