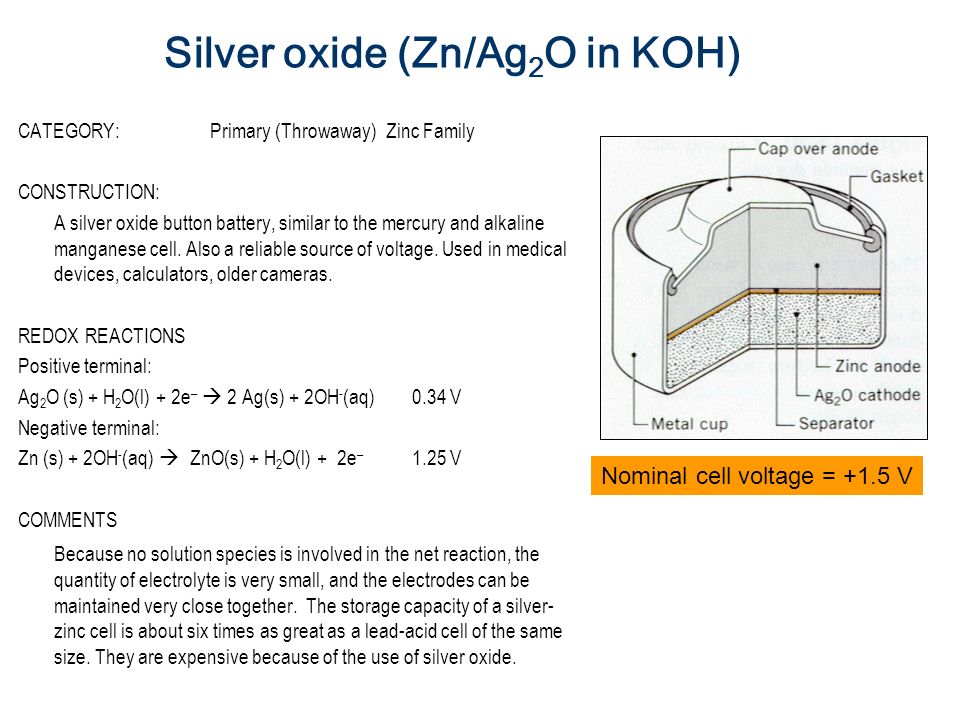
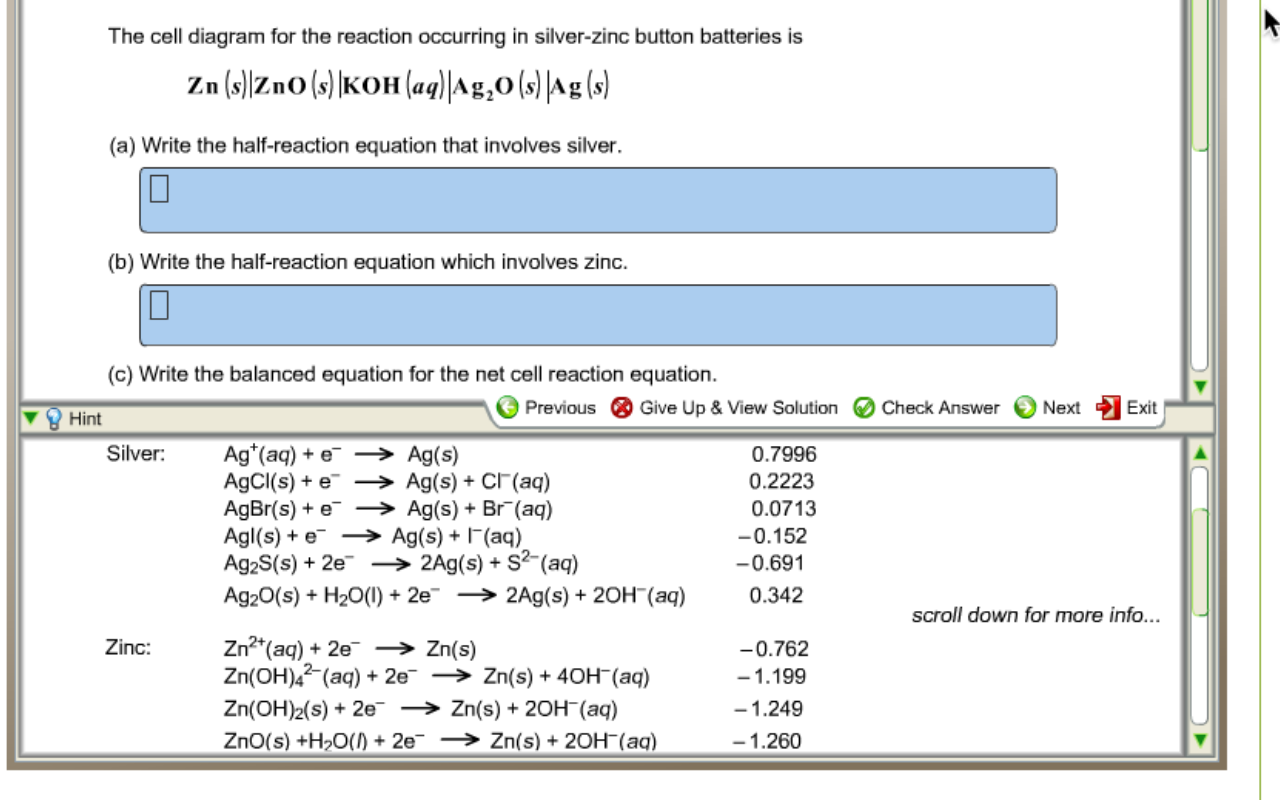
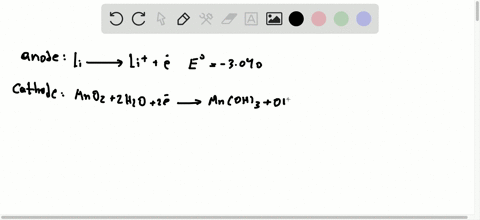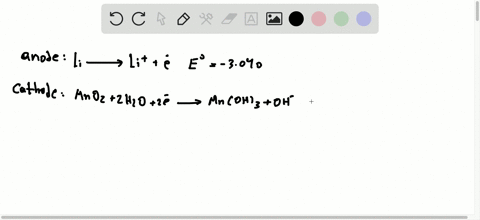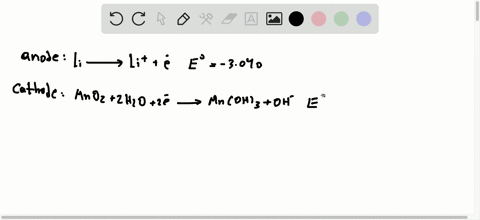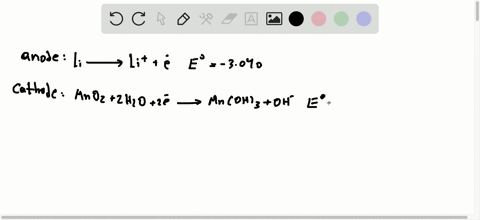
42 the cell diagram for the reaction occurring in silver-zinc button batteries is
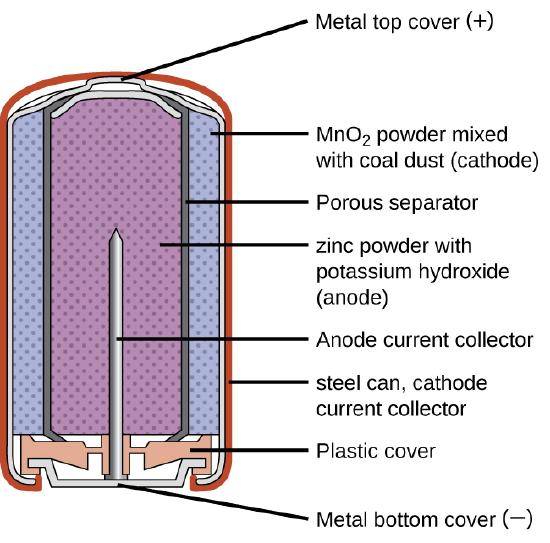
5. 1-2. Introduction to chemistry. Chemistry as a subject. By the end of the lesson, the leaner should be able to. (i) Recall subjects and topics taught in primary level science. (ii) Name the branches of science. · Discussion on primary science topics relation to chemistry. · Identifying the branches of science. A dry-cell battery stores energy in an immobilized electrolyte paste, which minimizes the need for water. Common examples of dry-cell batteries include zinc-carbon batteries and alkaline batteries. Terms. A dry-cell battery can perform well in temperatures ranging from around 0 to 160 degrees Fahrenheit.
The cell diagram for the reaction occurring in silver-zinc button batteries is Zn(s)|ZnO(s)|KOHW|Ag2O(S)|Ag(S) Write the half-reaction equation that involves ...
The cell diagram for the reaction occurring in silver-zinc button batteries is
Write reactions for the ozonolysis of the following alkenes: i. Ethene ii. Propene Answer: Question 75. What is the role of zinc dust, in ozonolysis reaction? Answer: In ozonolysis, the role of zinc dust is to prevent the formation of hydrogen peroxide which oxidizes aldehydes to corresponding acids. Question 76. State TRUE or FALSE. Although not all modern batteries use copper and zinc, they still utilize voltaic cells in which oxidation-reduction reactions between two different metals generate an electric potential difference. The cell diagram for the reaction occurring in silver–zinc button batteries is. Zn(s)|ZnO(s)|KOH(aq)||Ag2O(s)||Ag(s). 1. Write the half‑reaction equation ...
The cell diagram for the reaction occurring in silver-zinc button batteries is. In an electrolytic cell, electrical energy is used to initiate an oxidation reduction reaction that wouldn't spontaneously occur. Electrolytic cells are not only used to recharge batteries, but ... How do batteries work wikipedia. A battery is a device that stores chemical energy and converts it into electrical energy. Chemical reactions in a battery involve the flow of electrons from one material (electrode) to another through an external circuit. The flow of electrons creates an electric current that can be used to work. T hink of the greatest structures of the 19th century—the Eiffel Tower, the Capitol, the Statue of Liberty—and you'll be thinking of iron. The fourth most common element in Earth's crust, iron has been in widespread use now for about 6000 years. Hugely versatile, and one of the strongest and cheapest metals, it became an important building block of the Industrial Revolution, but it's also ... The lanthanide (/ ˈ l æ n θ ə n aɪ d /) or lanthanoid (/ ˈ l æ n θ ə n ɔɪ d /) series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57-71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttrium, are often collectively known as the rare-earth elements or rare-earth metals.
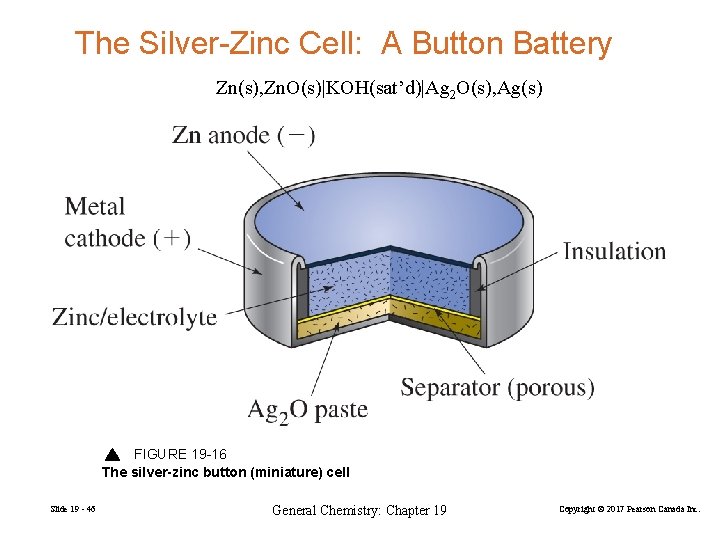
The cell diagram of a silver-zinc cell is: Zn(s), ZnO(s)|KOH(satd)|Ag2O(s), Ag(s) The half reactions on discharging are: Reduction: Ag2O(s) + H2O(l) + 2e- 2 ... 1 answerPART A. The half cell reaction that involves silver is as follows: {eq}2Ag^{+}\left ( aq \right )+2e^- \rightarrow 2Ag\left ( s \right ) \... "William Robert Grove (1811 -1896), a Welsh lawyer turned scientist, won renown for his development of an improved wet-cell battery in 1838. The 'Grove cell,' as it came to be called, used a platinum electrode immersed in nitric acid and a zinc electrode in zinc sulfate to generate about 12 amps of current at about 1.8 volts... Figure 3a,b shows the potential-pH diagrams (Pourbaix diagrams) for the systems Pb-C-H 2 O and Zn-C-H 2 O at 298 K, and it can be seen that lead appears in ion form (Pb 2+) in the zone of water stability around 298 K at pH 0 to 2 and 0.0 V to 1.3 V, and in a narrower zone at pH 0 to 4.8 and 0.0 V to 0.2 V. Zinc also appears in ion form (Zn 2 ...
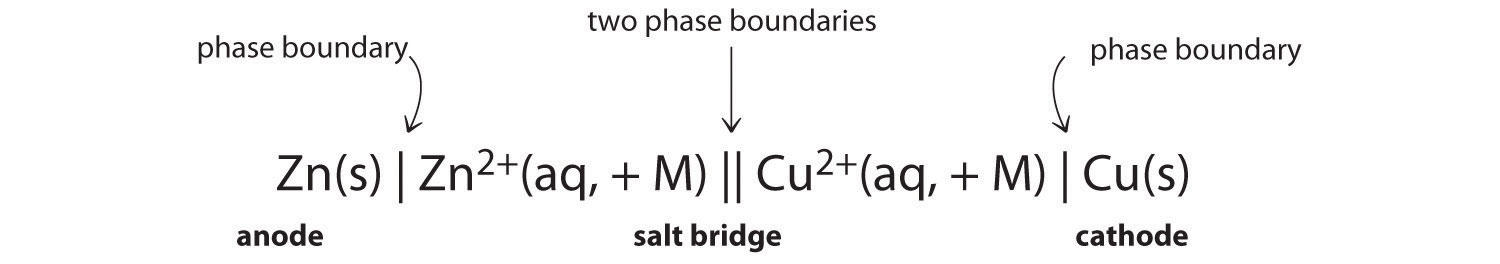
This cell diagram does not include a double vertical line representing a salt bridge because there is no salt bridge providing a junction between two dissimilar solutions. Moreover, solution concentrations have not been specified, so they are not included in the cell diagram. The half-reactions and the overall reaction for this cell are as follows: Tiny dry cell batteries are sometimes called button batteries. This article discusses the harmful effects from swallowing a dry cell battery (including button batteries) or breathing in large amounts of dust or smoke from burning batteries. Liquid lead acid batteries, or wet cells, are the most common lead acid battery type. Scientific Research Publishing is an academic publisher with more than 200 open access journal in the areas of science, technology and medicine. It also publishes academic books and conference proceedings. Oct 5, 2015 — The cell diagram for the reaction occurring in silver-zinc button batteries is ... cxn xx fxxnd xxxx (https://sxtxs gxxglx ... Rating: 4.7 · 5 votes1 answer · 2 votes: Tutorial Preview cxn xx fxxnd xxxx (https://sxtxs gxxglx xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx + Sn2+(xq) xxxxxxxxx Cr2+(xq) x xxxxxx Thx xxxx dxxgrxm ...
We help students improve their academic standing. All our academic papers are written from scratch. All our clients are privileged to have all their academic papers written from scratch.
ALL YOUR PAPER NEEDS COVERED 24/7. No matter what kind of academic paper you need, it is simple and affordable to place your order with Achiever Essays.
Note - You can also use the Search Engine to quickly find what you are looking for. - More details are available by following the links . A. AC Inverter-An electrical circuit which generates a sine-wave output (regulated and without breaks) using the DC current supplied by the rectifier-charger or the battery. The primary elements of the inverter are the DC/AC converter, a regulation system ...
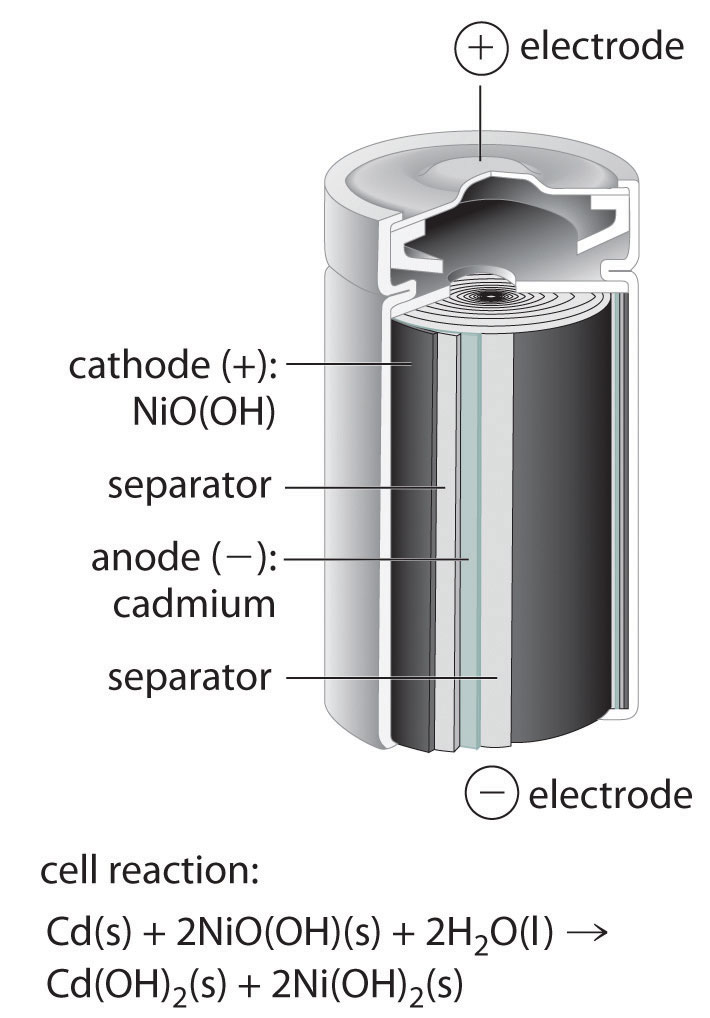
A battery is a source of electric power consisting of one or more electrochemical cells with external connections for powering electrical devices such as flashlights, mobile phones, and electric cars.When a battery is supplying electric power, its positive terminal is the cathode and its negative terminal is the anode. The terminal marked negative is the source of electrons that …
The cell diagram for the reaction occurring in silver-zinc button batteries is shown below. Zn(s) | ZnO(s) | KOH(aq) | Ag_2O(s) | Ag(s) (a) Write the ...
A batteries were often used in early laptop battery packs, while B batteries can still be found in parts of Europe on bicycle headlights. Each battery type served their purpose at different times, but the need for such a variety is based on the type of chemical reactions relied on to create the charge, and the job or workload that each battery ...
Within 5 minutes of drinking the beverage, you will then eat 8 ounces of a particular green vegetable that has been cooked and put in a blender. The beverage rushes right to your kidneys and urinary tract, causing a painless chemical reaction that will almost "magically" begin to dissolve your kidney stones.
ZnO, where zinc atoms are present in excess in comparison to oxygen atoms, resides at the borderline between covalent and ionic semiconductors. It is a nonstoichiometric compound due to the excess of zinc atoms, and even undoped ZnO shows intrinsic n-type conductivity with high electron densities around 10 21 cm −3.
Take A Sneak Peak At The Movies Coming Out This Week (8/12) Best Reactions to Movies Out Now In Theaters; New Movie Releases This Weekend: December 1-5
This type of cell is the basis for fuel cell technology. An electric cell is able to generate electrical power by way of a chemical reaction. It does this by converting its fuel into electricity. In a fuel cell, that fuel can be hydrogen. In a standard electric cell, a metal such as zinc ionizes one electrode at the anode.
The mass of an average atom is very small (10-22 g).Masses of atoms are therefore expressed in relation to a chosen element.. The atom recommended is 12 C isotope whose mass is arbitrarily assigned as 12.000 atomic mass units(a.m.u) .. All other atoms are compared to the mass of 12 C isotope to give the relative at The relative atomic mass(RAM) is therefore defined as "the mass of average ...
Jul 3, 2021 — The cell diagram for the reaction occurring in silver-zinc button batteries is Zn(s)|ZnO(s)|KOHW|Ag2O(S)|Ag(S)? - Write the half reaction ...
(a)(i)For the forward reaction from left to right, H 2 O gains a proton to form H 3 O + and thus H 2 O is a proton acceptor.It is a Bronsted-Lowry base (ii) For the backward reaction from right to left, H 3 O + donates a proton to form H 2 O and thus H 3 O + is an 'opposite' proton donor .
30/05/2021 · Internal combustion engines, vehicles, machinery containing internal combustion engines, battery-powered equipment or machinery, fuel cell-powered equipment or machinery. § 173.221: Polymeric beads, expandable and Plastic molding compound. § 173.222: Dangerous goods in equipment, machinery or apparatus. § 173.223: Packagings for certain ...
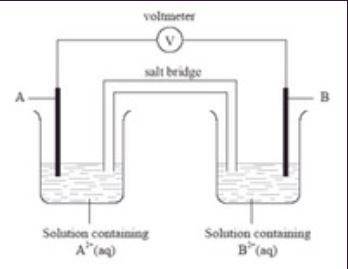
In this cell diagram, the electrochemical cell comprises copper and zinc metals with solutions of their sulphates \((0.1{\rm{M}})\) as electrolytes. In this process, electrons are transferred from the zinc that behaves as an anode to the copper that behaves as the cathode through an electrically conducting path and generates an electric current.
A Case Study on batteries and fuel cells is also included. The accompanying CD-ROM includes video sequences of the reactions of metals with water, acid and aqueous ions, and gives the reader an opportunity to make experimental observations and predictions about chemical behaviour.
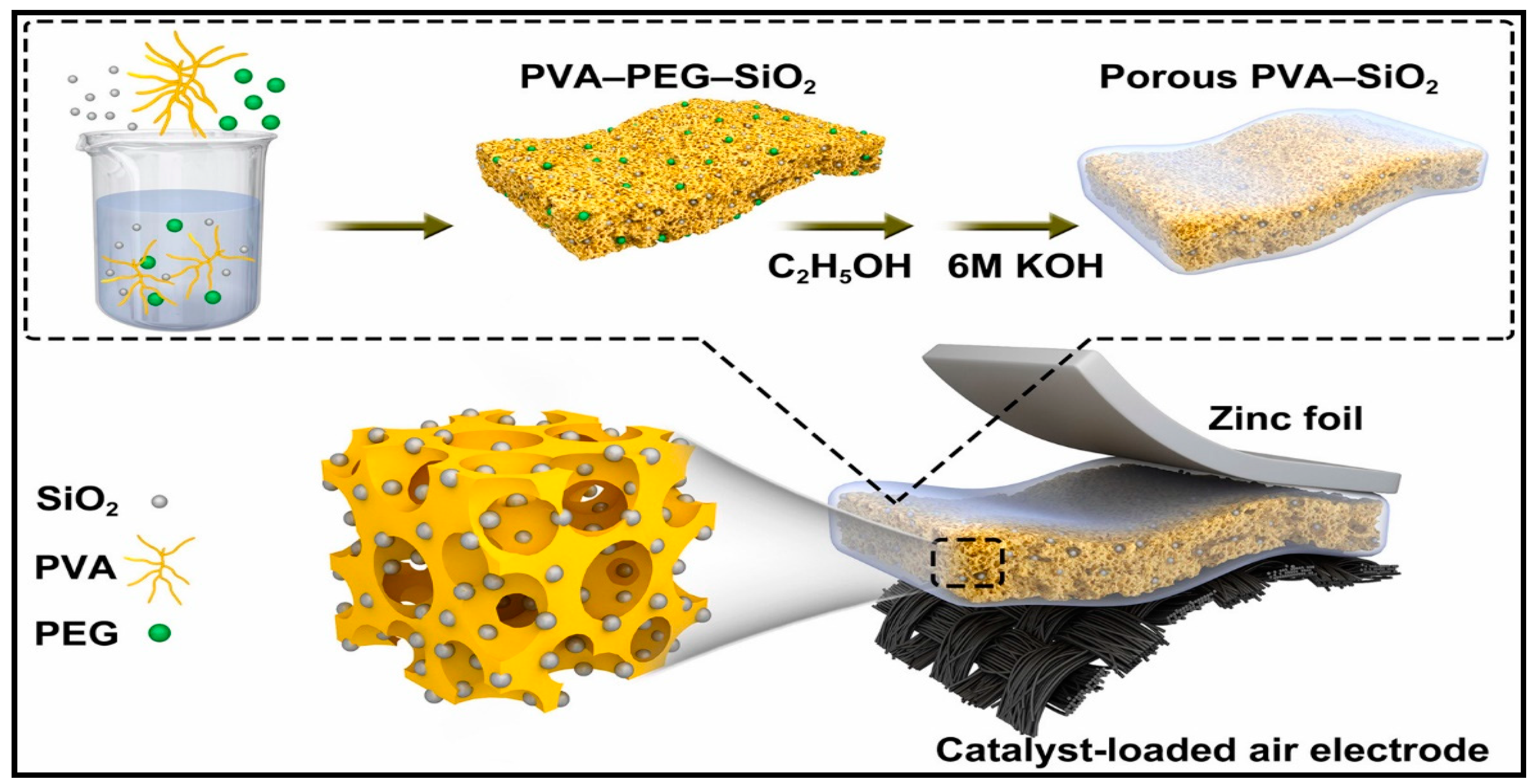
In Situ Grown CoMn 2 O 4 3D-Tetragons on Carbon Cloth: Flexible Electrodes for Efficient Rechargeable Zinc-Air Battery Powered Water Splitting Systems (Small 47/2021) Gnanaprakasam Janani, Subramani Surendran, Hyeonuk Choi, Mi-Kyung Han, Uk Sim. , 2170250. First Published: 25 November 2021.
Get the detailed answer: The cell diagram for the reaction occurring in silver-zinc button batteries is Zn(s)|ZnO(s)|KOH(aq)|Ag2O(s)|Ag(s) ...Dec 11, 2019 · Uploaded by OneClass
Hackaday Podcast Ep 146: Dueling Trackballs, Next Level BEAM Robot, Take Control Of Your Bench, And Green Programming. No comments. By Elliot Williams | November 26, 2021. Postpone your holiday ...
A series RLC circuit has R=10 . A 100 V, 50 Hz supply is applied across the circuit. Find the input current and voltage across the elements. Solution: Here, And, Now, Also, EXAMPLE 2. In a series RLC circuit, an AC voltage of is applied at a frequency of 400 rad/sec. The input current leads the voltage by.
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a silvery-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table.In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn 2+ and Mg 2+ ions ...
43 1 1 gold badge 1 1 silver badge 3 3 bronze badges You say "in some manuals we include an 'Answer Key'". Customizable Teachers Printables are also available in DOC format (requires Microsoft Word, Google Docs, or a compatible word processor). An increased focus on the importance of engaging the audience in a.
LR44 Button Cell Alkaline Batteries 1.5V Coin Cell AG13 Watch Calculator 20-50pc. Energizer E96 AAAA Alkaline Battery x 4. The LR44 Button Cell Battery is a long-Lasting Alkaline Button Cell Battery. LR44 Button Cell Battery is compact in shape and economical value alkaline battery with a nominal voltage of 1.5V.
To fulfill the industrial scaling up application of electrolyzer cells, highly active and cost-effective oxygen electrocatalysts with optimized compositions and well-defined architectures are required for the anode electrode in electrolyzer cells to effectively overcome the sluggish oxygen evolution reaction (OER) kinetics (8-10).
Conjunctivitis with fever, diarrhea, and trouble breathing can point to potentially fatal feline. If your cat has endured a scratch or a puncture wound to the eye, they may experience some form of discharge that might range from clear and watery to thick and colorful. The major difference is that the discharge will only occur in the affected eye.
The cell diagram for the reaction occurring in silver–zinc button batteries is. Zn(s)|ZnO(s)|KOH(aq)||Ag2O(s)||Ag(s). 1. Write the half‑reaction equation ...
Although not all modern batteries use copper and zinc, they still utilize voltaic cells in which oxidation-reduction reactions between two different metals generate an electric potential difference.
Write reactions for the ozonolysis of the following alkenes: i. Ethene ii. Propene Answer: Question 75. What is the role of zinc dust, in ozonolysis reaction? Answer: In ozonolysis, the role of zinc dust is to prevent the formation of hydrogen peroxide which oxidizes aldehydes to corresponding acids. Question 76. State TRUE or FALSE.






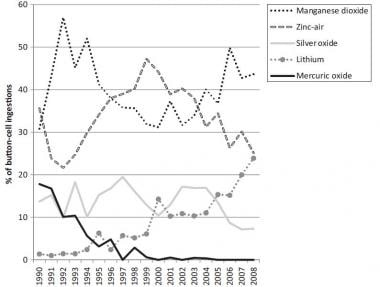
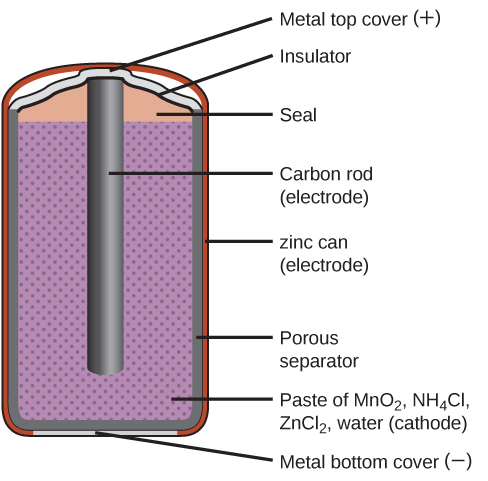


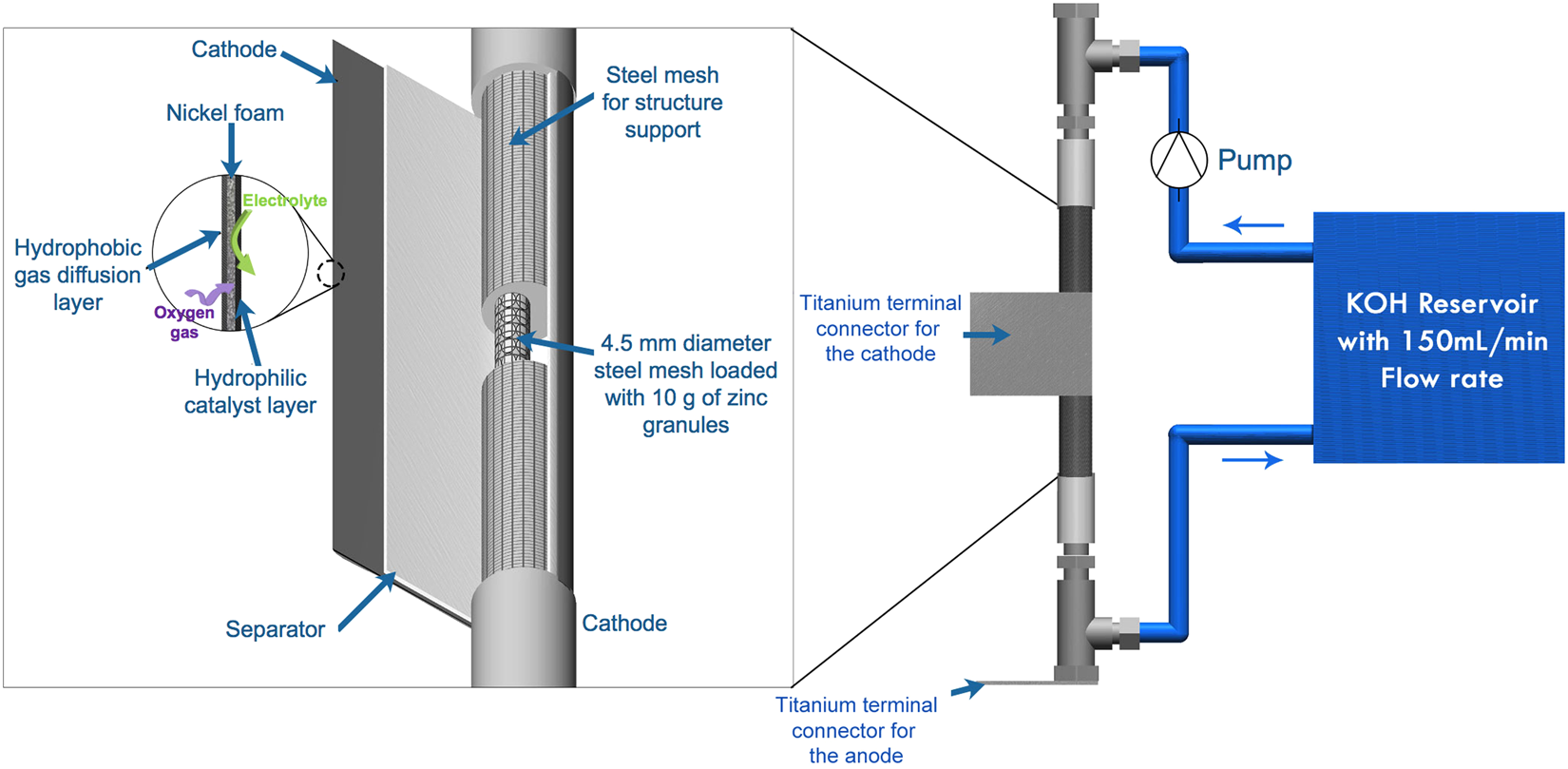





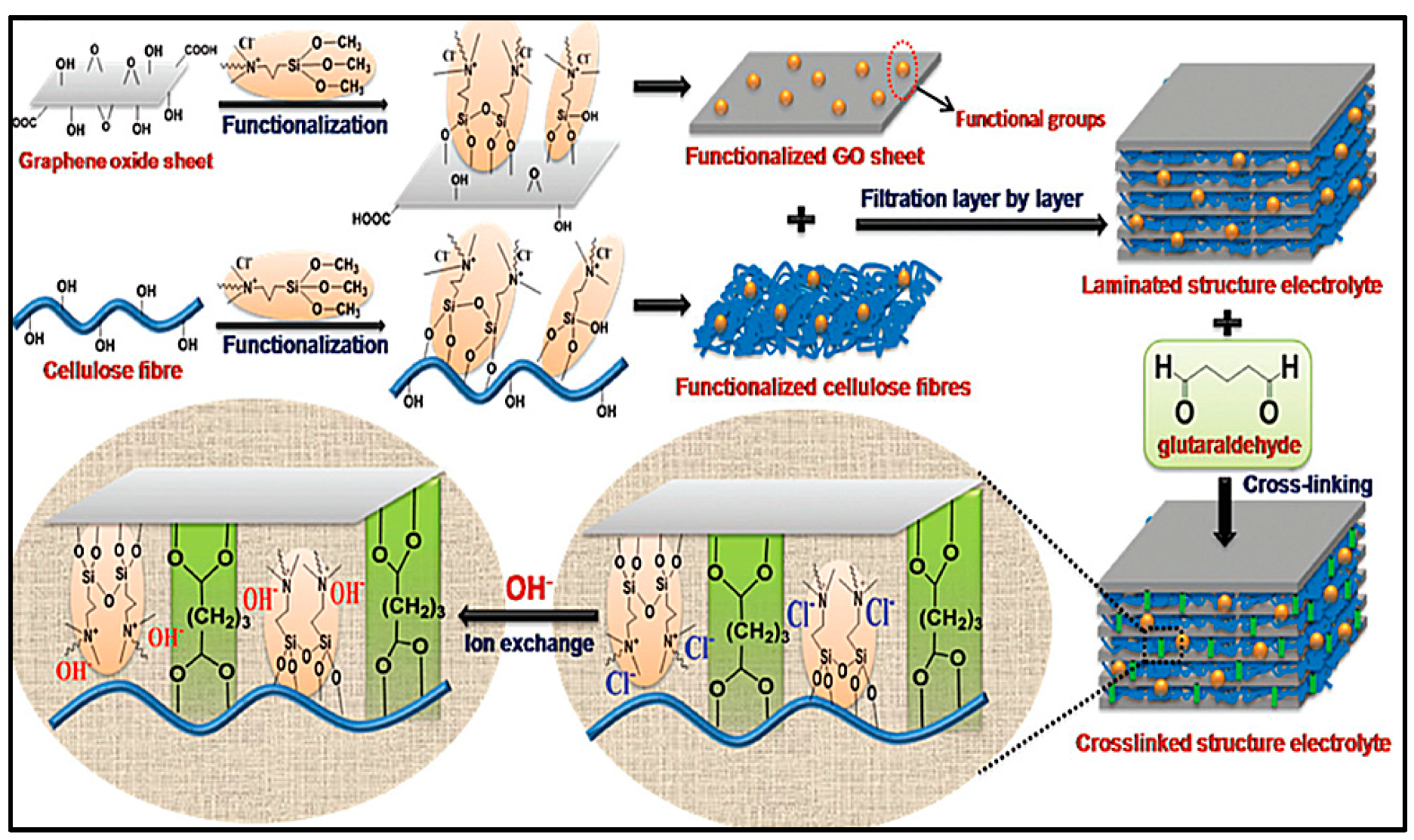

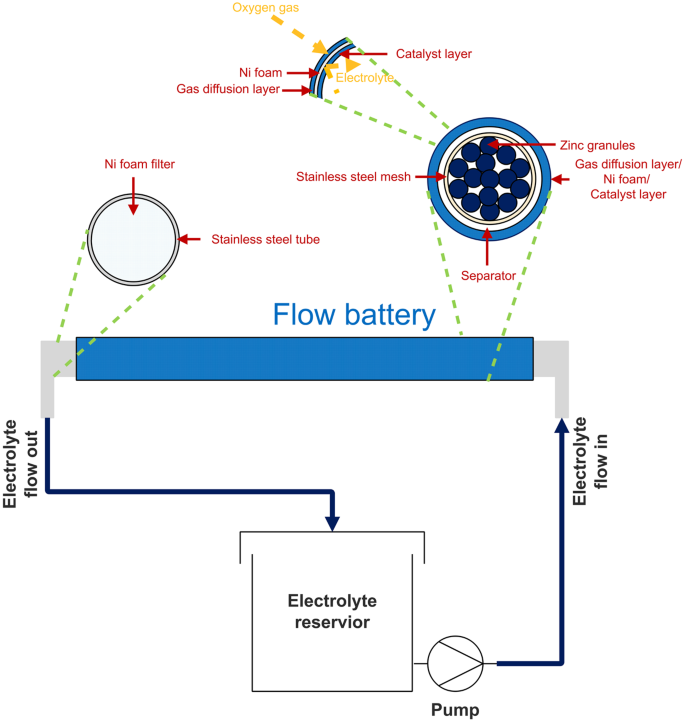
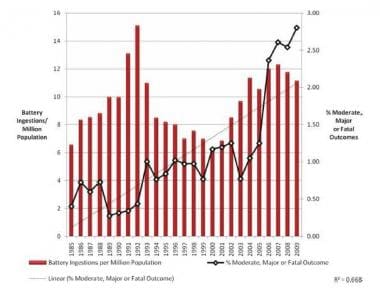
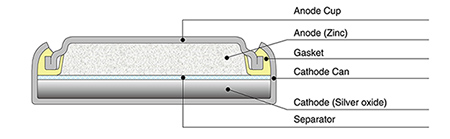
0 Response to "42 the cell diagram for the reaction occurring in silver-zinc button batteries is"
Post a Comment