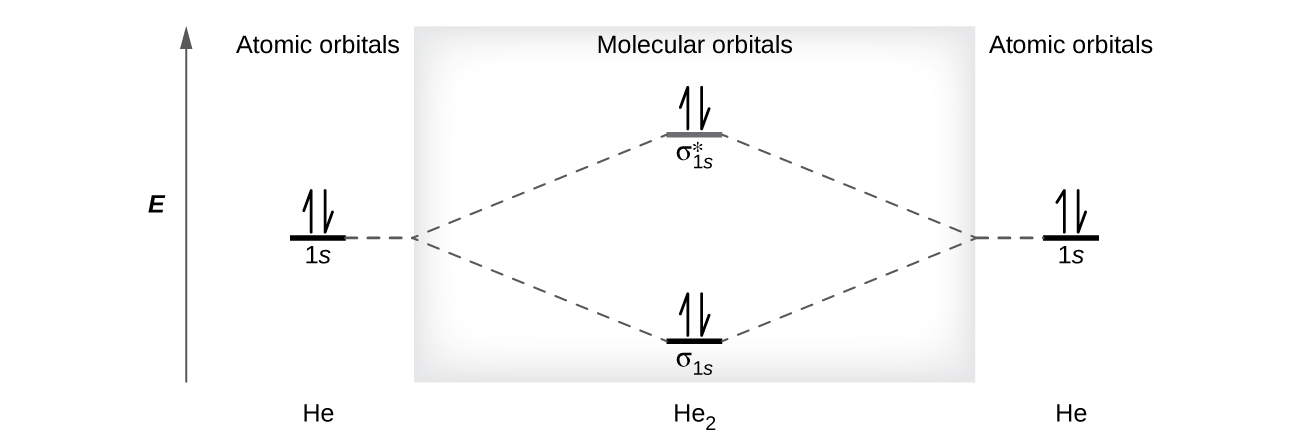
41 use the drawing of the mo energy diagram to predict the bond order of li2+.
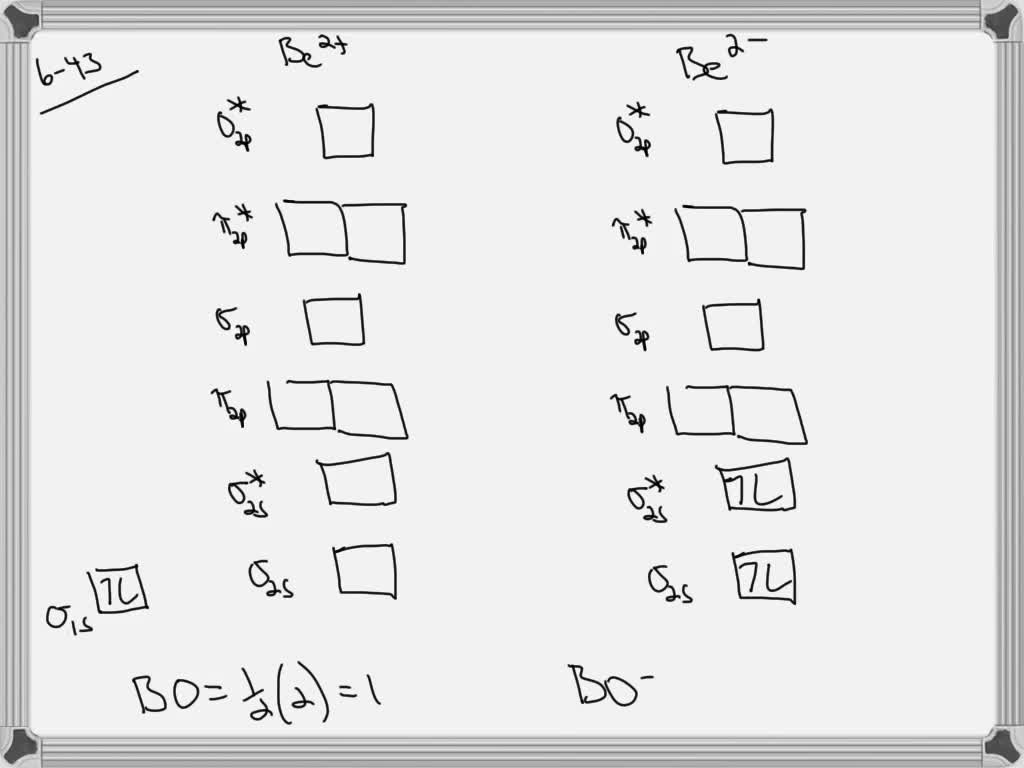
Answer to Draw an MO energy diagram and predict the bond order of Be2+ and Be2−. Do you expect these molecules to exist in the. From the above MO diagram we can see that number of elctrons in the bonding and antibonding orbital is same and hence Be does not form Be2 molecule(for. Part A. Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B. Use the drawing of the MO energy diagram to predict the bond order of Li2?.
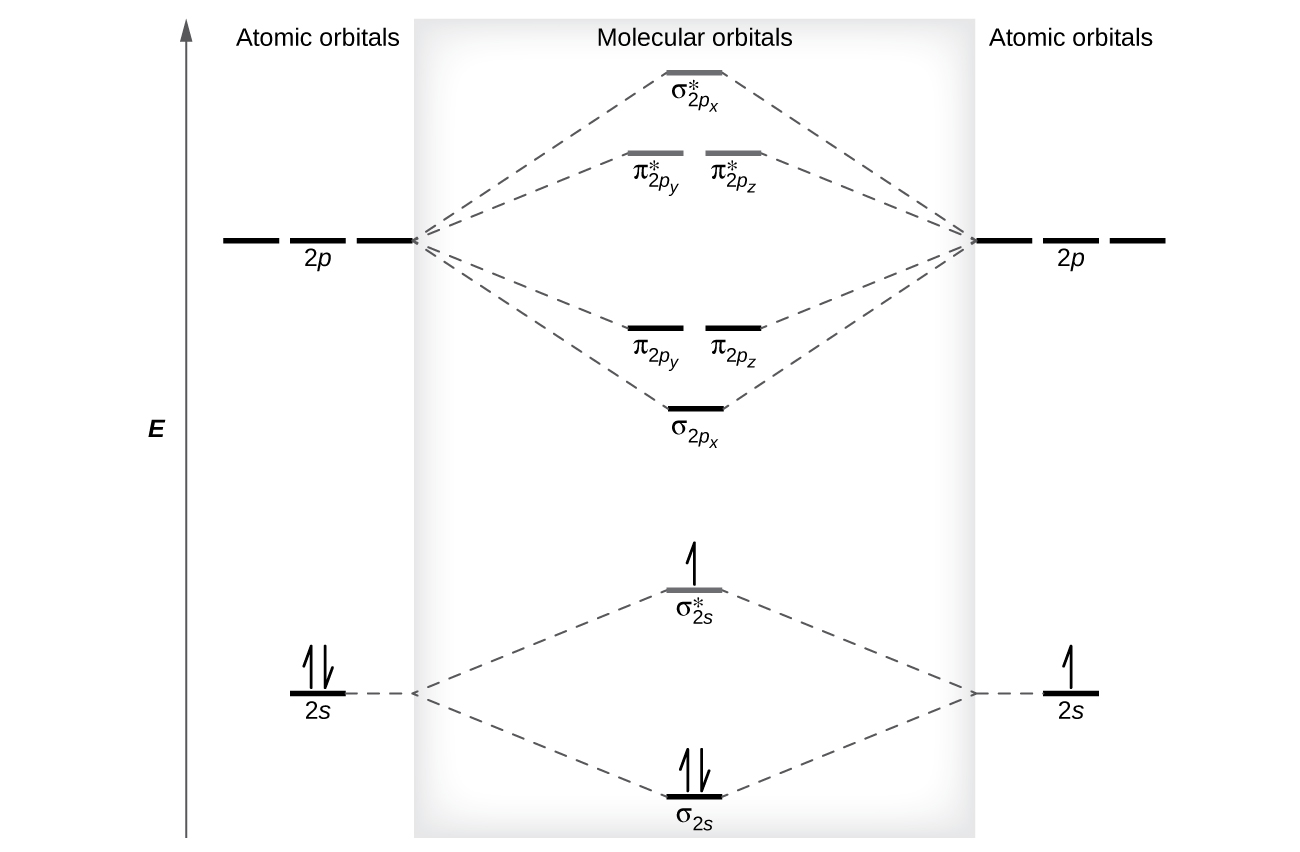
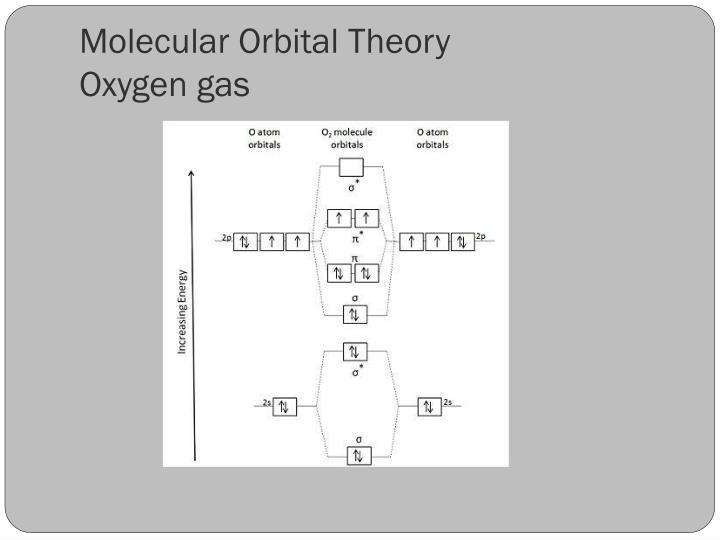
orbital. The electronic configuration for O 2 is: s 2s2 s* 2s2 s 2p2 p 2p4 p* 2p2. This electronic configuration indicates a bond order of 2, and the bond can be represented by O = O. There is no net bonding from the s 2s orbitals, because the number of bonding electrons equals the number of antibonding electrons.

Use the drawing of the mo energy diagram to predict the bond order of li2+.
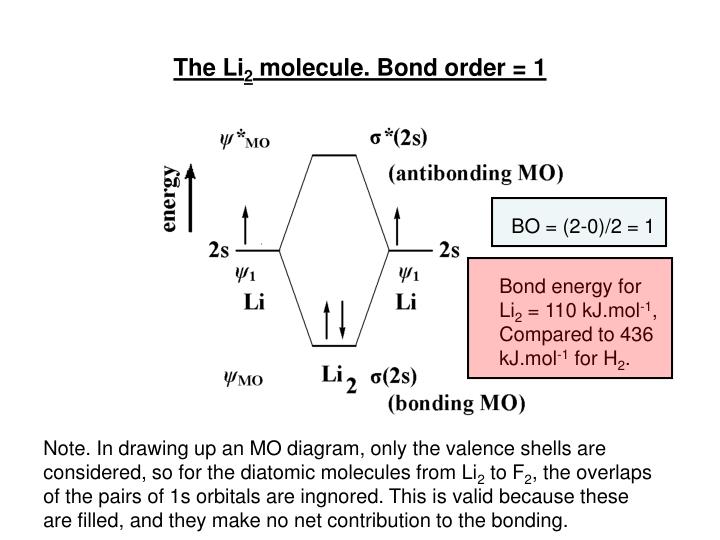
Li2 is more stable than Li+ 2, because the bond is (hypothetically) stronger (probably gas-phase). Here we consider the molecular orbital diagram (MO) of Li2: The bond order can be calculated in a simple manner. Just take electrons that are in each MO, and. for each electron in a bonding MO, it adds 0.5 to the bond order, because more bonding ... We can therefore use a molecular orbital energy-level diagram and the calculated bond order to predict the relative stability of species such as H 2 +. ... Use a molecular orbital energy-level diagram, such as those in Figure \(\PageIndex{2}\), ... Draw the molecular orbital energy-level diagram for the system. Use the drawing of mo energy diagram to predict the bond order ofli2 and li2. Draw the energy lebel diagram for o 2 2 with help of pen and paper and sho. Draw an molecular orbital energy diagram and predict the bond order of li2 and li2.
Use the drawing of the mo energy diagram to predict the bond order of li2+.. This chemistry video tutorial provides a basic introduction into molecular orbital theory. It describes the formation of bonding and antibonding molecular o... Draw the MO diagram for `O_2^+` This is a bit of a curveball, but a perfectly valid problem. ... The value in the bond order from MO diagrams is that we can now determine the number of bonds in between atoms that we otherwise would not be able to. ... With MO diagrams, we can predict the number of bonds in diatomic molecules. For example, here ... Answer to Draw a molecular orbital energy diagram for Li2. What is the bond order? Is the molecule likely to be stable? Explain. ... we would predict the Li2 molecule to be .CAcT Home Molecular orbitals of Li 2, Be 2, to F 2 Skills to develop. ... Describe the essential difference between a sigma and a pi molecular orbital. Define bond order ... Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation and the nitrosyl anion 1 Order of filling of molecular orbitals in heteronuclear diatomic molecules such as CO. Part A. Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B.
Problem: Use the drawing of MO energy diagram to predict the bond order of Be2+ and Be2–. Do you expect Be2– to exist in the gas phase? FREE Expert Solution. Bond Order = 1 2 [ # of e - in bonding MO - # of e - in antibonding MO] bonding MOs → without an asterisk ( e.g., σ 1s) antibonding MOs → those with an asterisk ( e.g., σ 1s* ). Answer (1 of 5): Bond order is the number of chemical bonds between a pair of atoms. Bond order is the number of chemical bonds between a pair of atoms; in diatomic nitrogen (N≡N) for example, the bond order is 3, while in acetylene (H−C≡C−H), the bond order between the two carbon atoms is 3 and... Molecular orbital energy level of Li2. diagram; the MOs go between them. Consider the MO diagram for Li2. This is a bond between two lithium atoms, which have an electron configuration of. The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital. How does bond order correspond to phase? Use the drawing of MO energy diagram to predict the bond order of [Be2]+ and [Be2]−. Determined that the bond order of [Be2]+ is (+1/2).
Using the molecular orbital energy ordering for second-row homonuclear diatomic molecules in which the p2p orbitals lie at higher energy than the s2p, draw MO energy diagrams and predict the bond order in a molecule or ion with each number of total valence electrons. Steps in predicting the hybrid orbitals used by an atom in bonding: 1. Draw the Lewis structure. 2. Determine the electron pair geometry using the VSEPR model. ... Construct the molecular-orbital energy-level diagram for a diatomic molecule or ion built from elements of the first or second row, and predict the bond order and number of unpaired ... MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine Chemistry questions and answers. Part A Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B Use the drawing of the MO energy diagram to predict the bond order of Li2?. Express the bond order as an integer or fraction. Part C Which molecules are predicted to exist ...
Use the drawing of mo energy diagram for co to predict the bond order. The bond order for be2 is calculated by. Draw the energy lebel diagram for o 2 2 with help of pen and paper and sho. Bond order indicates the stability of a bond. Answer to draw an mo energy diagram and predict the bond order of li2 and li2. C would this ion exist.
Welcome to Sarthaks eConnect: A unique platform where students can interact with teachers/experts/students to get solutions to their queries. Students (upto class 10+2) preparing for All Government Exams, CBSE Board Exam, ICSE Board Exam, State Board Exam, JEE (Mains+Advance) and NEET can ask questions from any subject and get quick answers by subject teachers/ experts/mentors/students.
4a1 is the σ* 2pz antibonding MO. To obtain the bond order, look at the molecular orbitals formed and decide whether they are bonding or antibonding. BO = 1 2 (bonding e− − antibonding e−) = 1 2 [(2 + 2 + 2 + 2) − (2 + 1)] = 2.5. And this should make sense because NO+ is isoelectronic with CO, which has a bond order of 3.
Answer (1 of 4): The atomic number of fluorine is 9, so a (neutral) F2 molecule has a total of 18 electron, or 14 valence electrons (excluding the four 1s electrons). The (F2)- ion has one more valence electron, or 15. The orbital diagram for a diatomic molecule is To find the bond order, add th...
Use the drawing of the MO energy diagram to predict the bond order of Li2+, and use the drawing of the MO energy diagram to predict the bond order of Li2−. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (30 ratings)
Use the drawing of MO energy diagram for CO to predict the bond order. (Use the energy ordering of O2) The two sigma bonds form between a hybrid sp orbital on Be and a p orbital on Br. Indicate which orbitals overlap to form the σ bonds in the following molecules: BeBr2.
In order to use hybrid-orbital analysis, we must already know the molecular geometry and bond angles in a molecule. ... Ammonia is both a donor and an acceptor of hydrogen in hydrogen-bond formation. Draw a diagram showing the hydrogen bonding of an ammonia molecule with two other ammonia molecules. ... Li2, Li2(+), Li2(-); Justify your choice ...
Draw an MO energy diagram and predict the bond order of Li2+and Li2-. Do you expect these molecules to exist in the gas phase? ... draw MO energy diagrams and predict the bond order in a molecule or ion with each number of total valence electrons. Will the molecule or ion be diamagnetic or
Problem: Draw the MO energy diagram for HCl on your own, then use it to predict the bond order for the molecule. FREE Expert Solution. 81% (79 ratings) FREE Expert Solution. First, identify the number of valence electrons. The number of valence electron per element is based on the group number.
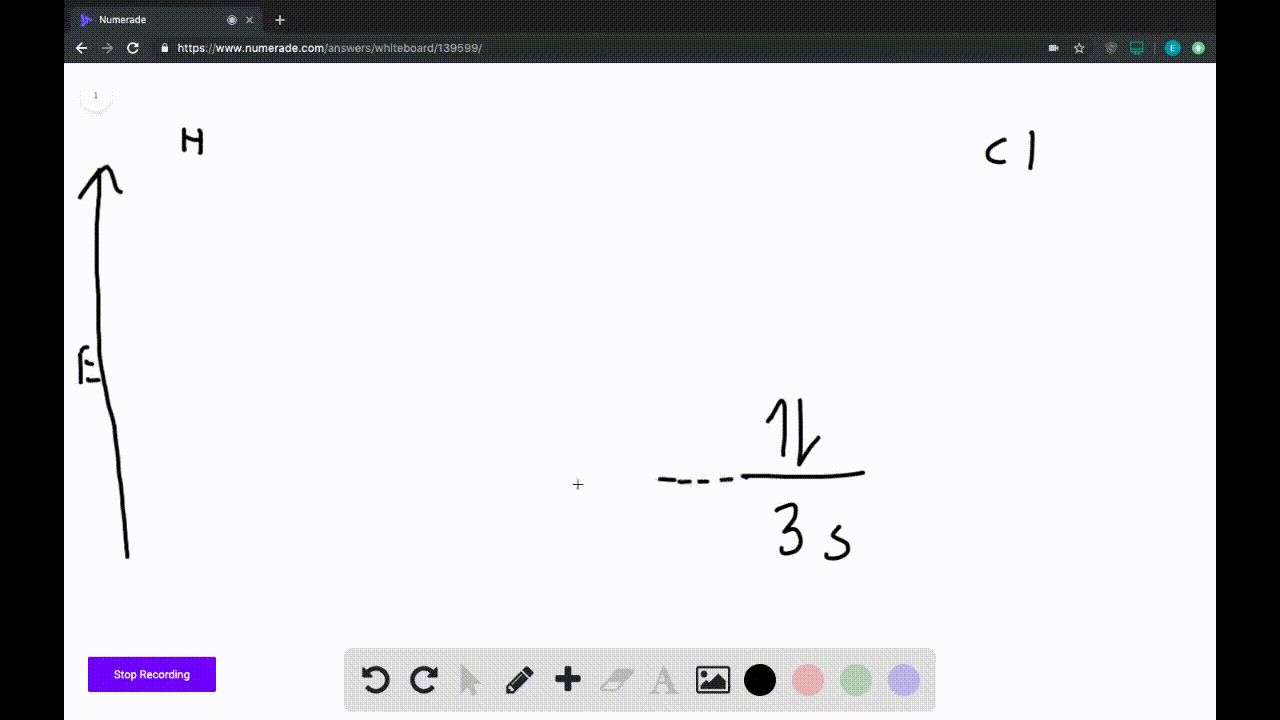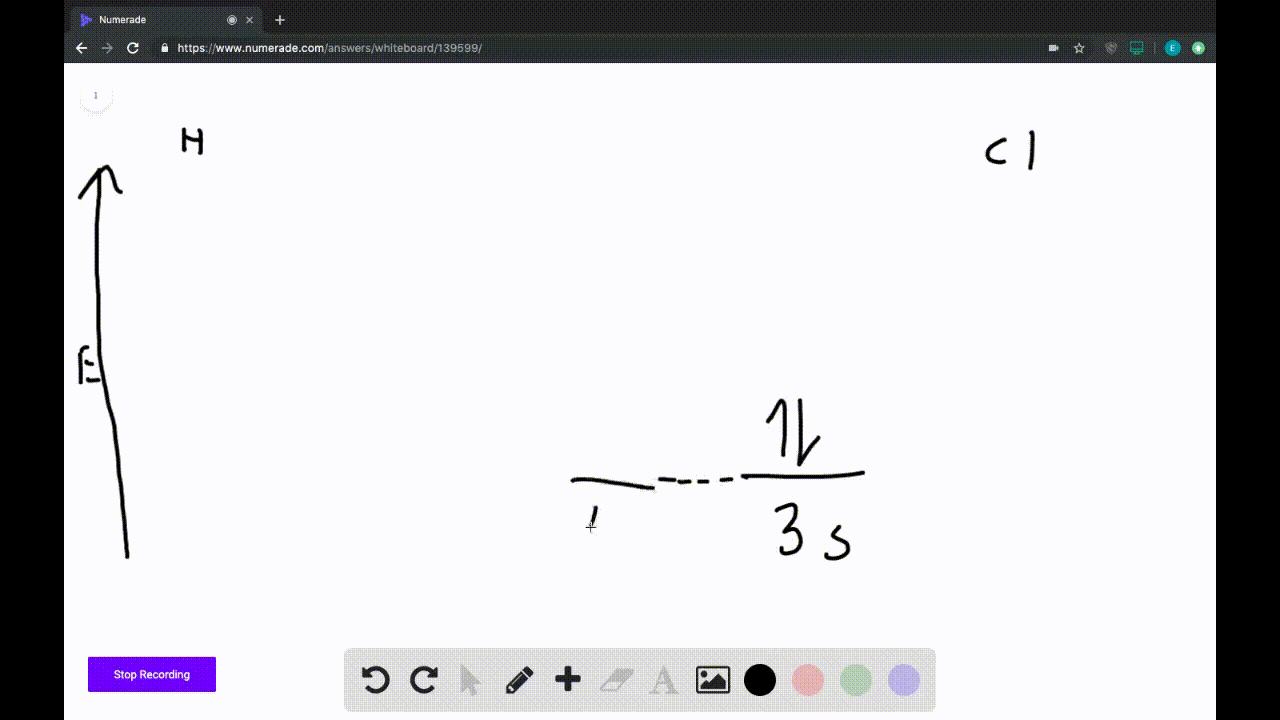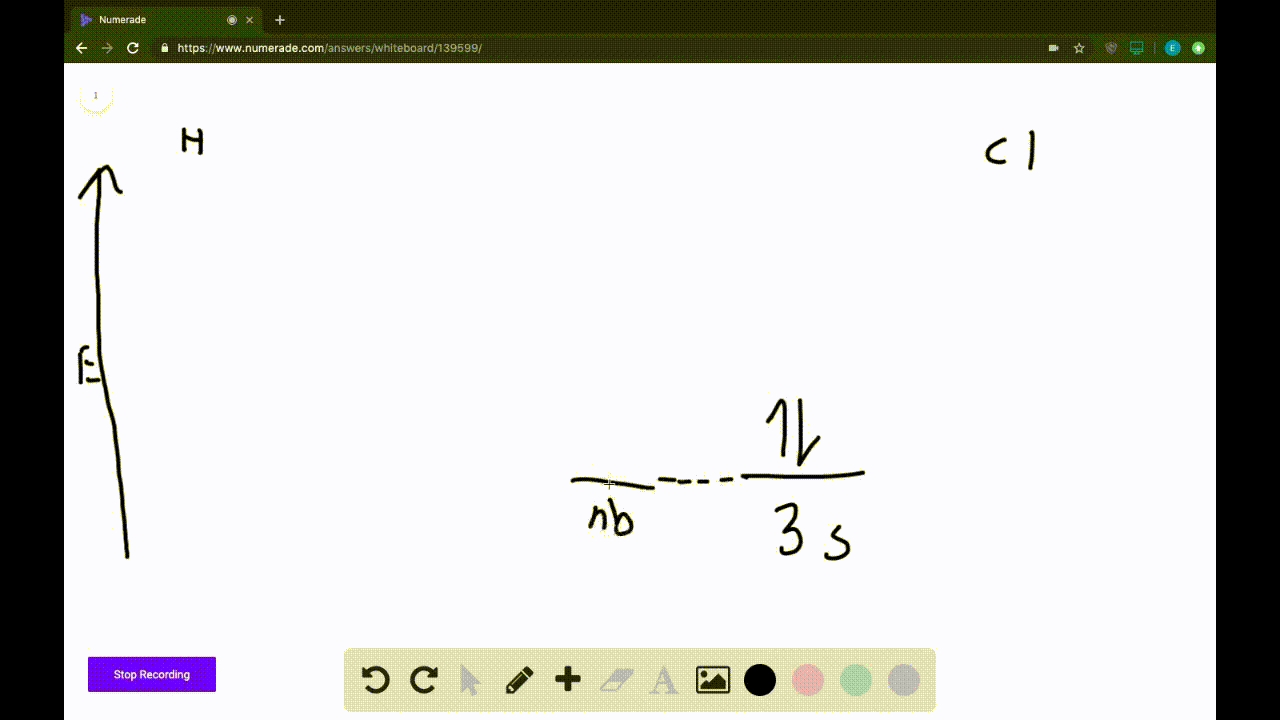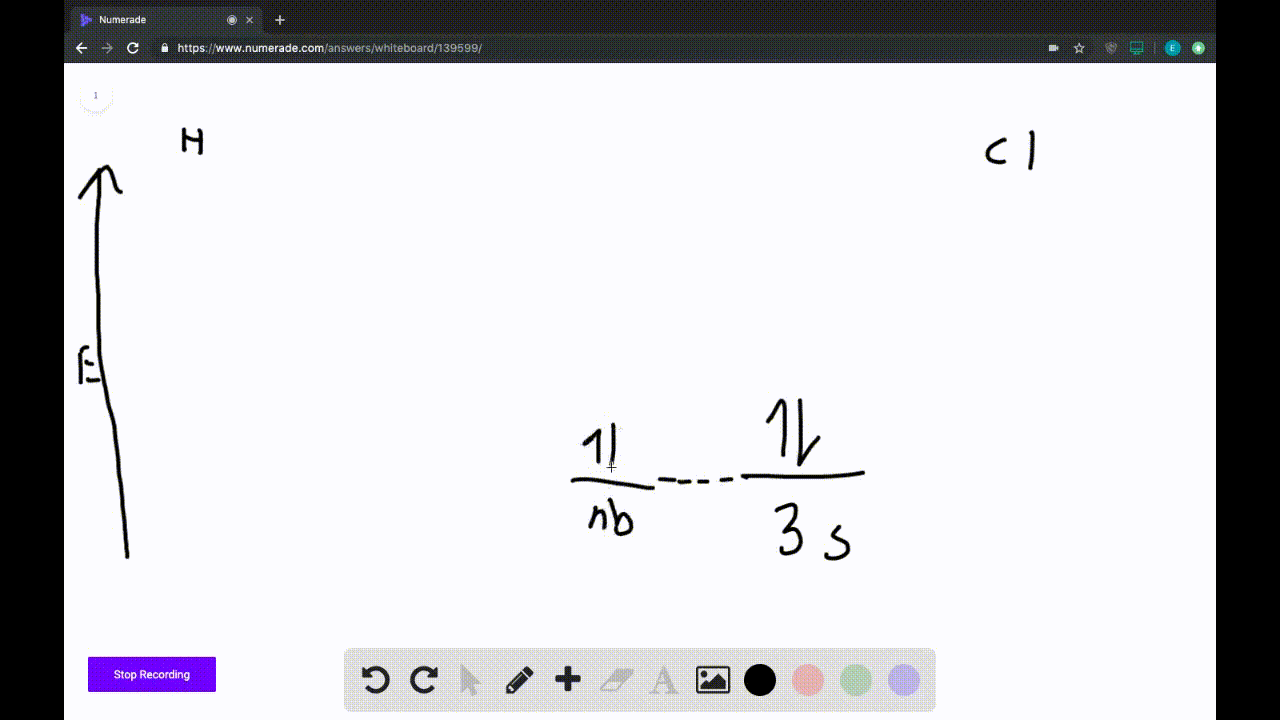
2) Stability of molecules in terms of bond order. Bond order is defined as half of the difference between the number of electrons present in the bonding and antibonding orbitals. Bond Order = ½ ( N b - Na) The molecule is stable if N b > Na ie. bond order is positive. The molecule is unstable if N b < Na i.e. the bond order is negative or zero.
Part AUse the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B Use the drawing of the MO energy diagram to predict the bond order of Li2?. Express the bond order as an integer or fraction. […]
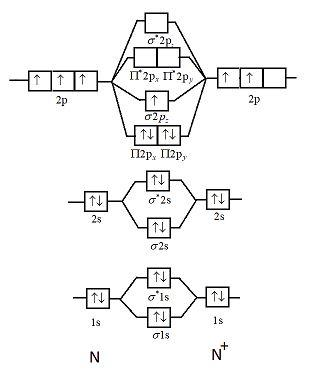
Use the molecular orbital diagram shown to determine which of the following is paramagnetic. Nitrogen can lose an electron to form N2+. Given the molecular orbital configuration of N2 [core] (σ2s)2 (σ *2s)2 (π2p)4 (σ2p)2 is N2+ diamagnetic or paramagnetic?
This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be . Learn to draw molecular orbital electron configuration energy diagrams. molecular orbital electron configuration diagram for Li2 (Figure "Molecular orbital.
Problem 43 Hard Difficulty. Draw an MO energy diagram and predict the bond order of Be2+ and Be2-. Do you expect these molecules to exist in the gas phase?
(i) Be2 molecule: The electronic configuration of Be(Z = 4) is: 4 Be 1s 2 2s 1 Be 2 molecule is formed by the overlap of atomic orbitals of both beryllium atoms. Number of valence electrons in Be atom = 2 Thus in the formation of Be 2 molecule, two outer electrons of each Be atom i.e. 4 in all, have to be accommodated in various molecular orbitals in the increasing order of their energies.
Use the drawing of mo energy diagram to predict the bond order ofli2 and li2. Draw the energy lebel diagram for o 2 2 with help of pen and paper and sho. Draw an molecular orbital energy diagram and predict the bond order of li2 and li2.
We can therefore use a molecular orbital energy-level diagram and the calculated bond order to predict the relative stability of species such as H 2 +. ... Use a molecular orbital energy-level diagram, such as those in Figure \(\PageIndex{2}\), ... Draw the molecular orbital energy-level diagram for the system.
Li2 is more stable than Li+ 2, because the bond is (hypothetically) stronger (probably gas-phase). Here we consider the molecular orbital diagram (MO) of Li2: The bond order can be calculated in a simple manner. Just take electrons that are in each MO, and. for each electron in a bonding MO, it adds 0.5 to the bond order, because more bonding ...
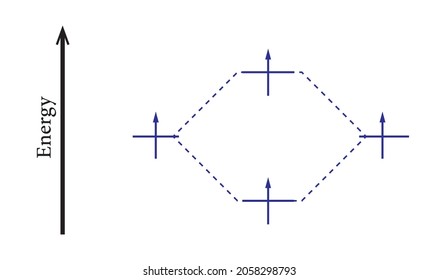
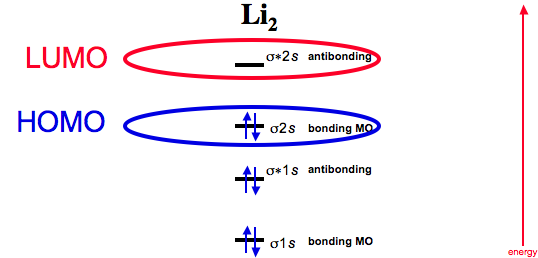


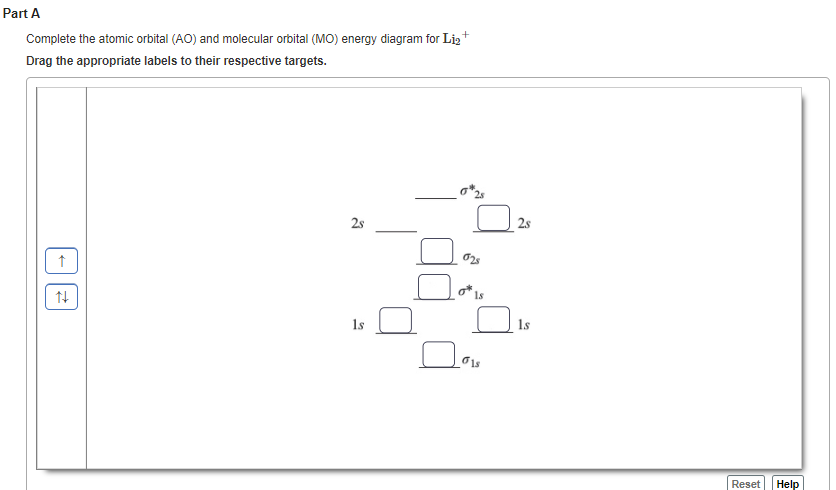

















0 Response to "41 use the drawing of the mo energy diagram to predict the bond order of li2+."
Post a Comment