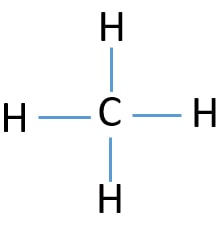
39 lewis diagram for ch4
Chemistry Q&A Library CH4 Sketch the proper Lewis structure for this substance, according to the guidelines given in this class. Be sure to follow octet/duet rules for each atom and use the total number of valence electrons available. Calculate the difference in electronegativity (AEN) for the bonds, being sure to use our electronegativity values. How to Draw the Lewis Dot Structure for CH4: MethaneA step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use...
Question: Draw the Lewis Structure for CH4. This problem has been solved! See the answer See the answer See the answer done loading. Draw the Lewis Structure for CH4. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.

Lewis diagram for ch4
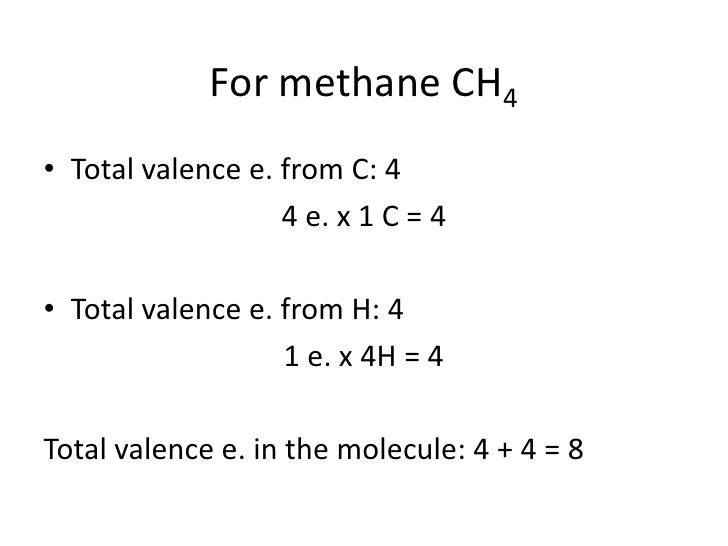
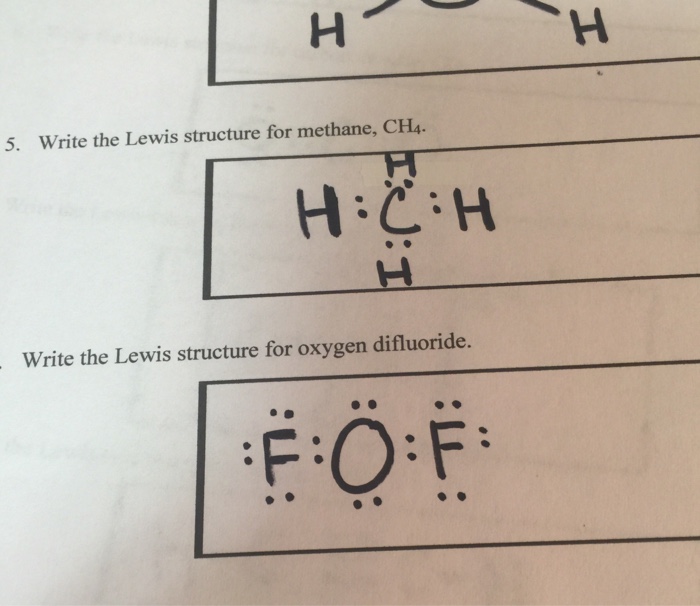
Answer: B carbon. Explanation. Lewis structure or dot structure is an easy way to get the bonding details of atoms in a molecule. If we talk about methane molecule carbon is central atom with four electrons that are bonded to four hydrogen atoms and each bond is single covalent bond.. Please see attached figure, For CH4 you have a total of 8 total valence electrons. Drawing the Lewis structure for CH4 (named methane) requires only single bonds. It's one of the easier ...28 Oct 2016 · Uploaded by Wayne Breslyn Lewis dot structure of CH 4. Alternatively a dot method can be used to draw the lewis structure. Calculate the total valence electrons in the molecule. C-4 H-1x4=4. Total=8. Put carbon in center and arrange hydrogen atoms on the diagramweb.nete electrons between carbon and hydrogen atoms. Dr.
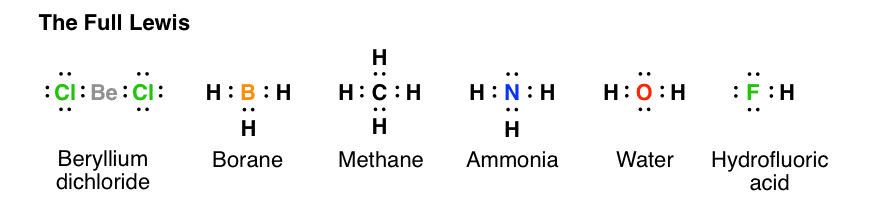
Lewis diagram for ch4. Methane (CH 4) Molecule Lewis Structure. Methane lewis structure contains four C-H bonds. There are no lone pairs in the valence shells of carbon atom. Carbon atom is the center atom and it is very easy to draw CH4 lewis structure. CH 4 lewis structure. There are following specifications in the lewis structure of methane. 3:49Craig Beals shows how to draw the Lewis Structure for Methane.This is a clip from the complete video ...15 Feb 2014 · Uploaded by Beals Science 2:45A step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure ...12 Jan 2021 · Uploaded by Wayne Breslyn A single Lewis structure can be used to represent the bonding in CH4.There are 2 equivalent Lewis structures for nitryl chloride where the double bond to oxygen can be placed on either of the ...
1:41Hey Guys,In this video we are going to learn about the Lewis structure of CH4.It is a chemical formula for ...20 Feb 2021 · Uploaded by Geometry of Molecules 2:02How to Draw the Lewis Structure of CH4 (methane) · Comments • 22.27 Jul 2019 · Uploaded by chemistNATE 0:59Lewis Dot Structure of CH4 (methane) ... I quickly take you through how to draw the Lewis Structure of ...1 Oct 2011 · Uploaded by kentchemistry.com In addition. Lewis Structures for CH4. Step-by-step tutorial for drawing the Lewis Structure for CH4. Lewis Dot Structure for CH4 #2 Find the number of “octet” electrons for the molecule. C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 . We show two ways to draw the CH4 Lewis structure, methane.
So in the Lewis structure of CH4 or Methane, there are four single or covalent bonds between each Hydrogen and Carbon atom. There are four ...25 Mar 2021 · Uploaded by Geometry of Molecules CH4 Lewis Structure, Molecular Geometry, and Hybridization. Methane or CH4 is a naturally occurring gas and relatively abundant on the Earth, making it an economically efficient fuel. As it releases more light and heat on burning, it is preferred more than coal, fossil fuel, or gasoline for energy production. It is one reason why overproduction ... Lewis dot structure of CH 4. Alternatively a dot method can be used to draw the lewis structure. Calculate the total valence electrons in the molecule. C-4 H-1x4=4. Total=8. Put carbon in center and arrange hydrogen atoms on the diagramweb.nete electrons between carbon and hydrogen atoms. Dr. For CH4 you have a total of 8 total valence electrons. Drawing the Lewis structure for CH4 (named methane) requires only single bonds. It's one of the easier ...28 Oct 2016 · Uploaded by Wayne Breslyn
Answer: B carbon. Explanation. Lewis structure or dot structure is an easy way to get the bonding details of atoms in a molecule. If we talk about methane molecule carbon is central atom with four electrons that are bonded to four hydrogen atoms and each bond is single covalent bond.. Please see attached figure,

The Lewis Structure For Methane Ch4 Is Shown How Many Valence Electrons Does Each Hydrogen Atom In Brainly Com

Draw The Lewis Structure For Methane Ch4 And Ethane C2h6 In The Box Below Then Predict Which Brainly Com

Draw An Electron Dot Diagram To Show The Formation Of Each Of The Following Compounds Methane Chemistry Shaalaa Com

Chemistry Lewis Structure Valenzstrichformel Structural Formula Molecule Methane Electron Number Lewis Structure Valenzstrichformel Chemistry Png Pngwing
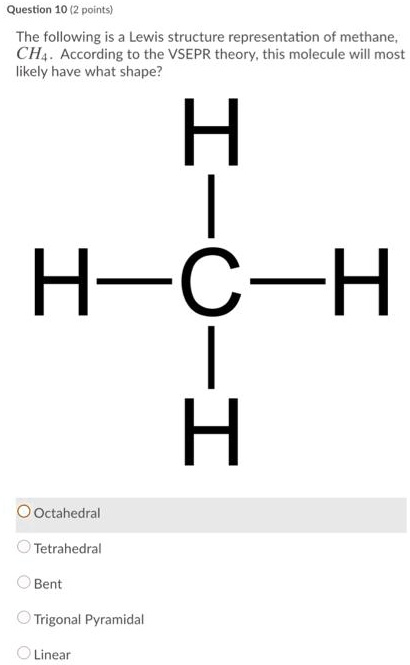
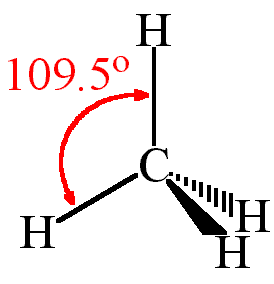
Solved Question 10 Polnts The Following Is Lewis Structure Representation Of Methane Ch4 According To The Vsepr Theory This Molecule Will Most Likely Have What Shape H H C H A Octahedral Tetrahedral Bent Trigonal

What Is The Molecular Formula Of Methane Draw The Lewis Structure And Determine The Hybridization Of The Central Atom Study Com
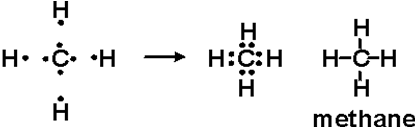
Answer The Following Question Explain The Bonding In Methane Molecule Using Electron Dot Structure From Chemistry Chemical Bonding Class 10 Icse
Explain The Bonding In Methane Molecule Using Electron Dot Structure Sarthaks Econnect Largest Online Education Community














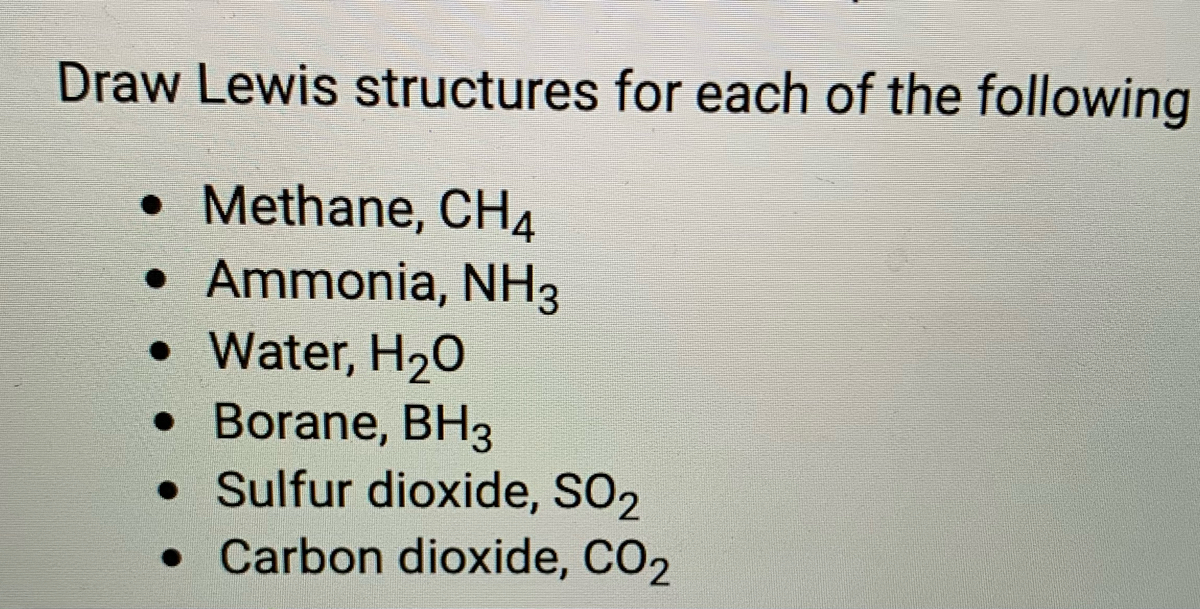


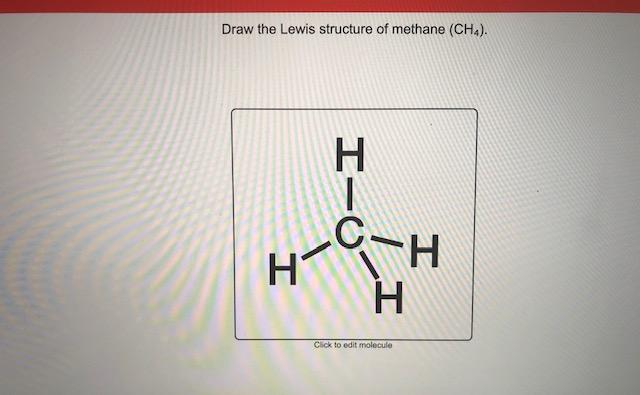
0 Response to "39 lewis diagram for ch4"
Post a Comment