38 ground state orbital diagram
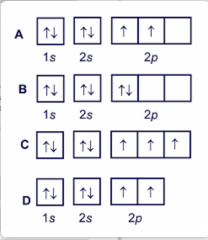
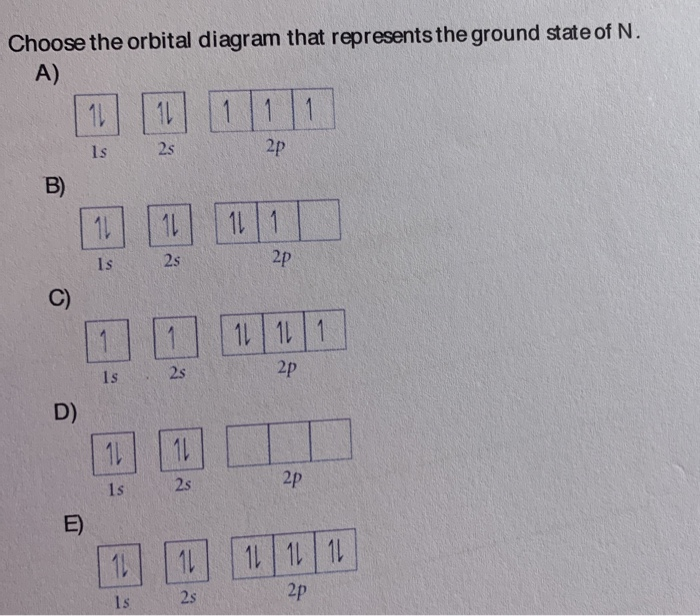
Things to Memorize Polyatomic Ion list to Memorize How to remember polyatomic ions Strong Acids & Bases to Memorize Metric System Conversions Location of Charges to be Memorized Choose the orbital diagram that represents the ground state of N. Learn this topic by watching The Electron Configuration Concept Videos All Chemistry Practice Problems The Electron Configuration Practice Problems
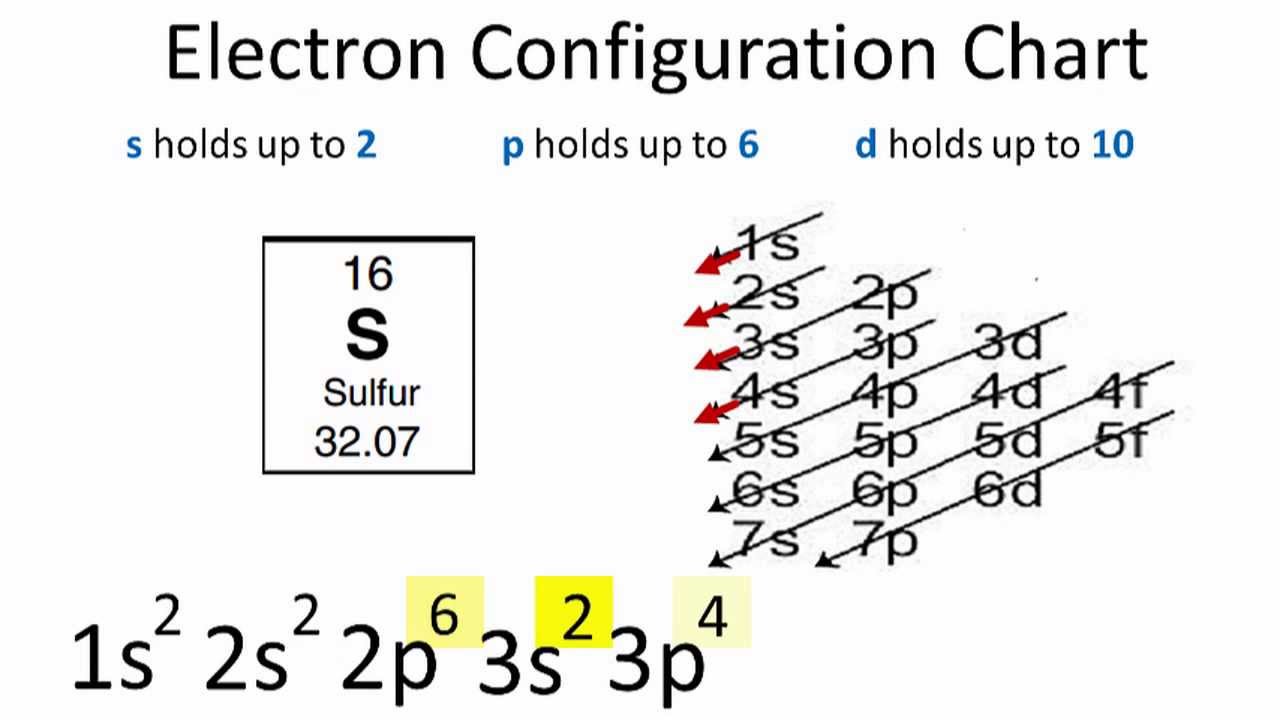
The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining four electrons. Therefore the sulfur electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 4.
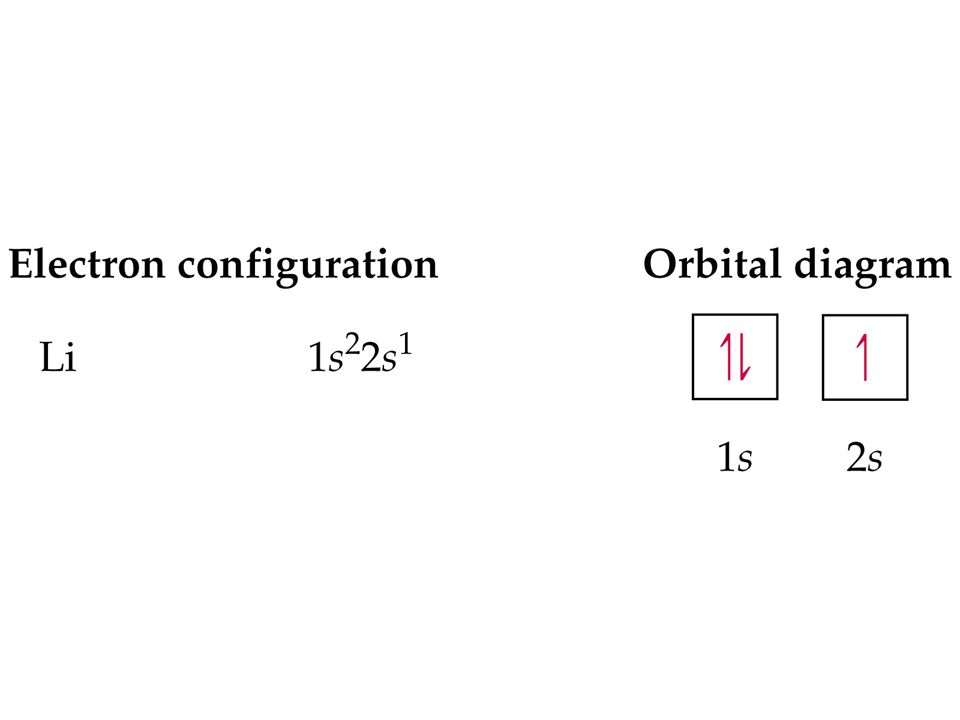
Ground state orbital diagram
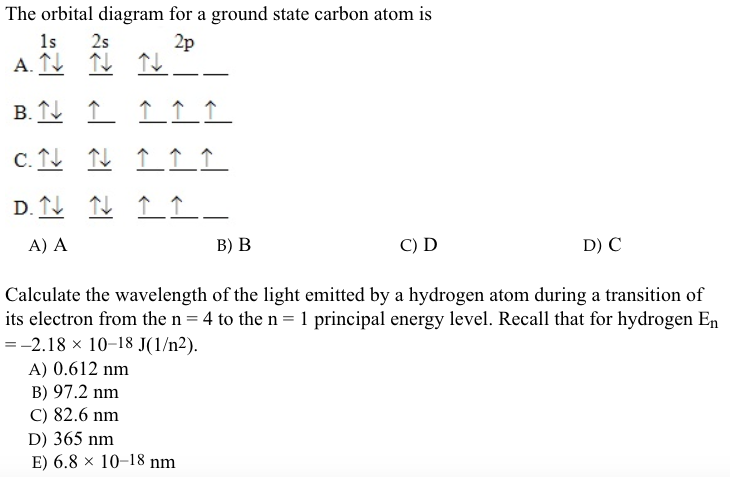
Figure %: The ground state electron configuration of carbon, which has a total of six electrons. The configuration is determined by applying the rules of the Aufbau Principle. Valency and Valence Electrons The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. What is a ground state orbital diagram? The ground state electron configuration is the arrangement of electrons around the nucleus of an atom with lower energy levels. The electrons occupying the orbitals of varying energy levels naturally falls towards the lowest energy state or ground state. The arrangement of electrons in the atomic orbitals of an atom is called the electron configuration. Electron configurations can be determined using a periodic table. ... You should be familiar with how to determine an electron configuration for an atom and identify the valence electrons. You should be able to identify both ground ...
Ground state orbital diagram. Arrows are drawn inside the boxes to represent electrons. Two electrons in the same orbital must have opposite spin so the arrows are drawn pointing in opposite directions. The following is an orbital diagram for selenium. Oct 15, · Draw the orbital diagram for the ground state of Selenium. Choose the orbital diagram that represents the ground state of N. Choose the valence orbital diagram that represents the ground state of Zn. Give the ground state electron configuration for Se. [Ar]4s23d104p4. Give the ground state electron configuration for I. [Kr]5s24d105p5. The approximate order of filling of atomic orbitals, following the arrows from 1s to 7p. (After 7p the order includes subshells outside the range of the diagram, starting with 8s.) The principle works very well (for the ground states of the atoms) for the known 118 elements, although it is ... However, the experiment has shown that the electron configuration of Palladium is: 46P d:1s2,2s2,2p6,3s2,3p6,4s2,3d10,4p6,5s0,4d10. The answer is more complicated than a student at the AP chemistry level would understand, I will just give couple of reasons that will simplify the question: Full d orbitals are more stable than partially filled ones.
After the 4s is full we put the remaining four electrons in the 3d orbital and end with 3d4. Therefore the expected electron configuration for Chromium will be 1s 2 2s 2 2p 6 3s 2 3p 4 4s 2 3d 9. Note that when writing the electron configuration for an atom like Cr, the 3d is usually written before the 4s. 1.9A: Ground state electronic Configuration. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. From the electrons in an atom, to the differing orbitals and hybridization, the ground state electron configuration sheds light on many different atomic properties. Writing Orbital Diagrams. We will now construct the ground-state electron configuration and orbital diagram for a selection of atoms in the first and second periods of the periodic table. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons. We ... July 14, 2020 - Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with the loss of or gain of electrons in their subsequent orbitals.
Here is a schematic orbital diagram for a hydrogen atom in its ground state: Figure \(\PageIndex{1}\): One electron in. From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number n and their value of l ( s , p , d , or f ), with the ... Orbital Diagram 1s ↿⇂ 2s ↿⇂ 2p ↿⇂ ↿⇂ ↿⇂ 3s ↿⇂ 3p ↿⇂ ↿⇂ ↿⇂ 3d ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ 4s ↿⇂ 4p ↿ ↿ ↿ 4d 4f: ... Which element has the ground state electron configuration as KR 4d2 5s2? That would be technetium, element 43. Orbital Diagram For Vanadium (V) | Vanadium Electron Configuration. ... Ground State Electron Configuration of V. The most stable arrangement or the lowest energy is referred to as the ground state configuration. When we talk about the ground state electronic configuration firstly, it is important to know the electronic configuration of the ... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
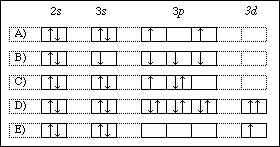
Classify each orbital diagram for ground-state electron configurations by the rule or principle it violates. Drag the appropriate items to their respective bins. View Available Hint(s) Reset Help Aufbau violation |2p 11 Hund violation Pauli violation 2p 20 1 11 1s 3d 3p 11 11 4p 11 11 11 58 11 Submit Previous Answers Request Answer X Incorrect ...
Determine the electron configuration of Se using the concept of orbital diagram and electron configuration · Before we can do that, we have to first write the electron configuration of a neutral ground state selenium (Se).
orbital diagram for sodium confirms that the 3s sublevel is lower in energy than the 3p sublevel. The s sublevel is located lower on the page than the p sublevel. 10. The lowest potential energy arrangement of electrons in an atom is called the ground state. Ground state electron configurations can be predicted by a strict set of rules known as the
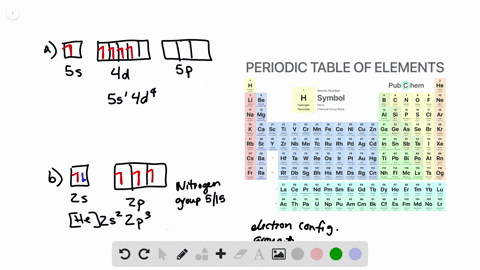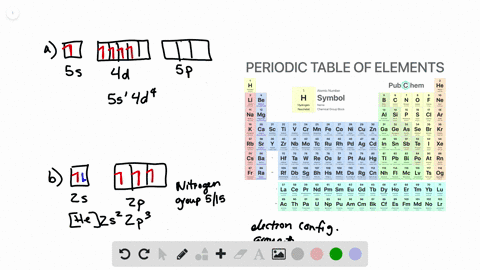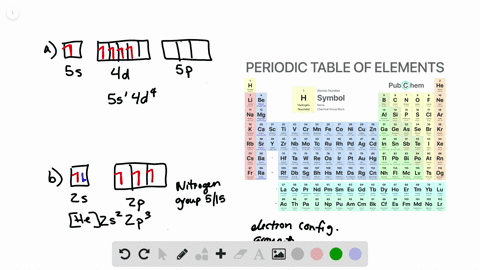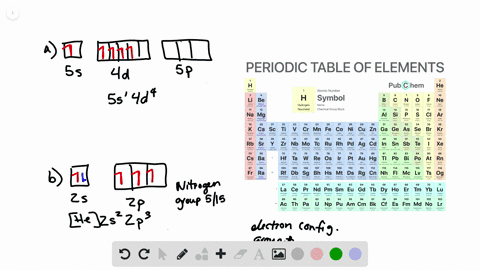
Solved Draw Atomic Orbital Diagrams Representing The Ground State Electron Configuration For Each Of The Following Elements A Na B Co C Kr How Many Unpaired Electrons Are Present In Each Element
That is, in a ground-state atom, all electrons are in the lowest possible energy levels. eg: Consider a carbon atom whose electron configuration is the following. The total energy of the electrons in this carbon atom can not be lowered by transfering one or more electrons to different orbitals.
December 14, 2016 - The ground state is 1s^2 2s^2 2p^2. In the explanation below, I show a common means of diagramming this. Using arrows to show the spin orientation of each electron, the orbital diagram is often shown this way: The single electrons in the two p-orbitals is in accordance with Hund's Rule.
Answer to Classify each orbital diagram for ground-state electron configurations by the rule or principle it violates....
0 Comments. on Orbital Diagram For Germanium. orbital. Because an electron can have either one of two spins, any orbital can hold a maximum of four . The orbital diagram for germanium is. 1s. 2s. 2p. 3s. Oxidation States, +4,2. Electrons Per Shell, 2 8 18 4. Electron Configuration, [Ar] 3d10 4s2 4p2. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2.
We will now construct the ground-state electron configuration and orbital diagram for a selection of atoms in the first and second periods of the periodic table. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons.
Complete the atomic orbital diagram for the ground-state electronic configuration of chlorine. Atomic Orbitals: An Atomic Orbital represents the place where there is a maximum probability of ...
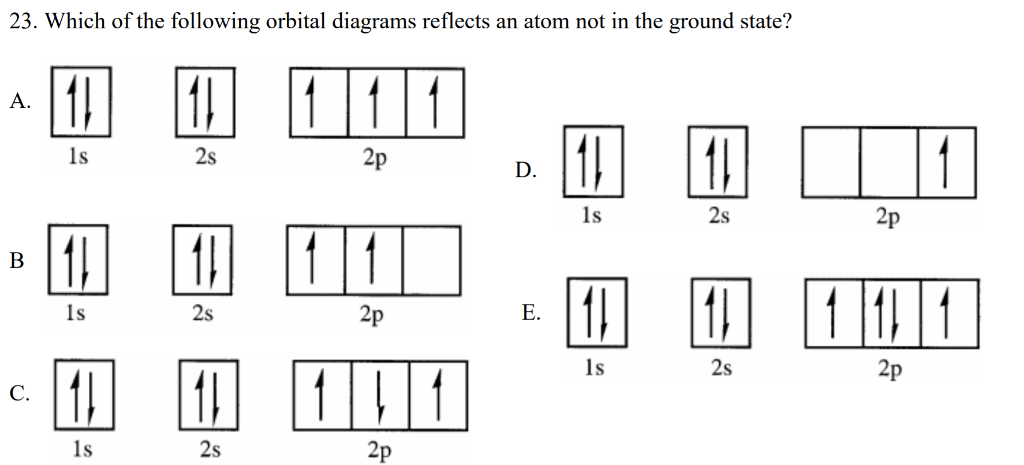
The above diagram roughly depicts the relative energy difference between these three ways of filling 2 electrons into the three p orbitals. Ground State: 1s22s22p x 12p y 1 (or 1s22s22p x 12p z 1 or 1s22s22p y 12p z 1) [CAUTION: these don’t explicitly state the electron’s spin!]

Electron Configurations Pogil What Is The Electron Structure In An Atom Interactive Worksheet By Leasa Slater Wizer Me
orbital diagram for sodium confirms that the 3s sublevel is lower in energy than the 3p sublevel. 10. The lowest potential energy arrangement of electrons in an atom is called the ground state. Ground state electron configurations can be predicted by a strict set of rules known as the Aufbau principle ("aufbau"means filling up). Examine the ...
This WebElements periodic table page contains properties of free atoms for the element selenium
FREE Answer to 16) Choose the valence orbital diagram that represents the ground state of Se2-.
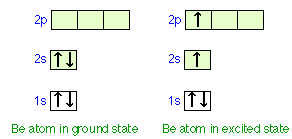
For this reason, carbon will form an excited state by promoting one of its 2s electrons into its empty 2p orbital and hybridize from the excited state. By forming this excited state, carbon will be able to form four bonds. The excited state configuration is shown below:
The electron configuration for oxygen is: 1s^2 2s^2 2p^4 This video will walk you through the step of writing orbital diagram. The video uses Kr as an example, but the process is exactly as the same as what you need to do for oxygen. Hope this helps!
The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows. Energy levels: 2, 8, 6 Orbitals: 1s2 2s2 2p6 3s2 3p4 If you need to fill in the little boxes, here's one for you. Each arrow represents one electron.
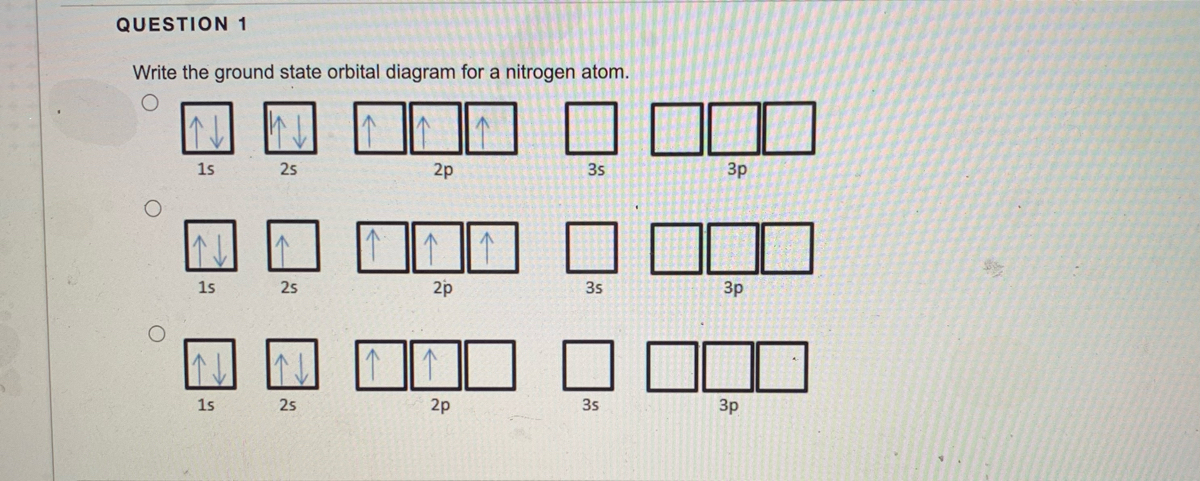
Ground State Electron Configuration For Nitrogen. When we talk about the electronic configuration, then the ground state Nitrogen Electron Configuration is written as 1s 2 2s 2 2p 3.Below you can get the full image representation which will help you to understand the topic more easily.
That is, in an excited-state atom not all electrons are in the lowest possible energy levels. eg. Consider a carbon atom whose electron configuration is the following. The total energy of the electrons in this carbon atom can be lowered by transfering an electron from a 2P orbital to the 2S orbital.
The ground state electron configuration is the arrangement of electrons around the nucleus of an atom with lower energy levels. The electrons occupying the orbitals of varying energy levels ...
Answer (1 of 5): Just use the Aufbau principle. Bromine has an atomic number of 35, so that's 35 electrons. Start writing it out...1s2(remember the 2 is an exponent/superscript), 2s2, 2p6, so on... You'll end up with 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5 or [Ar] 4s2 3d10 4p5 , whichever you choose,...
3 weeks ago - Hund’s Rule states that orbitals of the same energy, those which differ only in their orientation, are filled with electrons with the same spin before the second electron is added to any of the orbitals. This is why electrons have up spin, ↑, in the orbital diagrams of B to N and of Al ...
Let's put all these stuff into play, how this all come together. Okay let's do the orbital diagram for iron, iron we know is on its ground state of 26 electrons, so we know the first electrons are going to go into the 1s orbital and we said 2 electrons can fall into the 1s orbital.
For the orbital diagrams (with the arrows showing the paired/parallel electrons), the ground state electron configuration of an atom would only be the configuration made by the Aufbau principle (and Pauli's/Hund's). Any other configuration (with different numbers of electrons occupying different orbitals) would be the excited state.
February 14, 2019 - We will now construct the ground-state electron configuration and orbital diagram for a selection of atoms in the first and second periods of the periodic table. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement ...
August 5, 2021 - Hund, 1896–1997), which today ... configuration of carbon, which is 1s2 2s2 2p2, is understood to correspond to the orbital diagram shown in c. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons....
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine

6 A Write An Orbital Diagram For The Ground State Of Magnesium Mg And Phosphorous P Marks 1 6 B Which Of The Following Elements Would Be Expected To Be Paramagnetic
Ground state orbital diagrams and electron configuration by Elysia Barajas - October 15, 2014
The arrangement of electrons in the atomic orbitals of an atom is called the electron configuration. Electron configurations can be determined using a periodic table. ... You should be familiar with how to determine an electron configuration for an atom and identify the valence electrons. You should be able to identify both ground ...
What is a ground state orbital diagram? The ground state electron configuration is the arrangement of electrons around the nucleus of an atom with lower energy levels. The electrons occupying the orbitals of varying energy levels naturally falls towards the lowest energy state or ground state.
Figure %: The ground state electron configuration of carbon, which has a total of six electrons. The configuration is determined by applying the rules of the Aufbau Principle. Valency and Valence Electrons The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons.

















0 Response to "38 ground state orbital diagram"
Post a Comment