38 molecular orbital diagram for he2
PDF 26. Correlation Diagrams: H and He Molecular Orbitals 26. Correlation Diagrams: H 2 and He 2 Molecular Orbitals Below is the correlation diagram for two hydrogen atoms and the resulting H 2 molecule. Each atom has one electron before bonding. When the two hydrogen atoms bond, those two electrons occupy one of the two molecular orbitals that were created. 1) How does the energy of the two electrons ... Molecular orbital correlation diagrams for He2, He2+, N2 ... Molecular orbital correlation diagrams for He2, He2+, N2, N2+, CO, and CO+. After a preliminary check with He2 and He2+, self‐consistent field calculations have been carried out for the nitrogen and carbon monoxide molecules and some of their positive ions for the range of internuclear distances from about 1.5 times equilibrium down to 0.01 bohr.
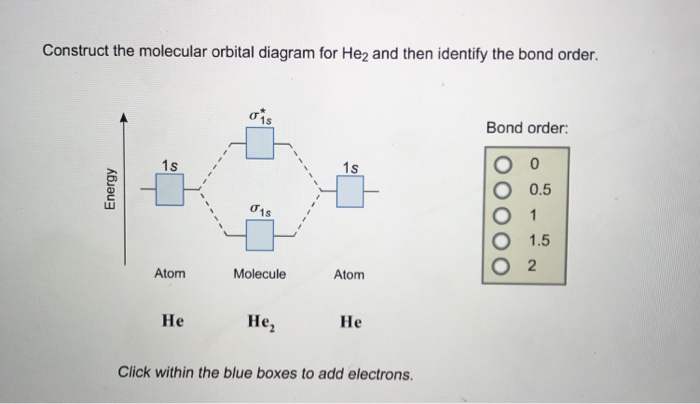
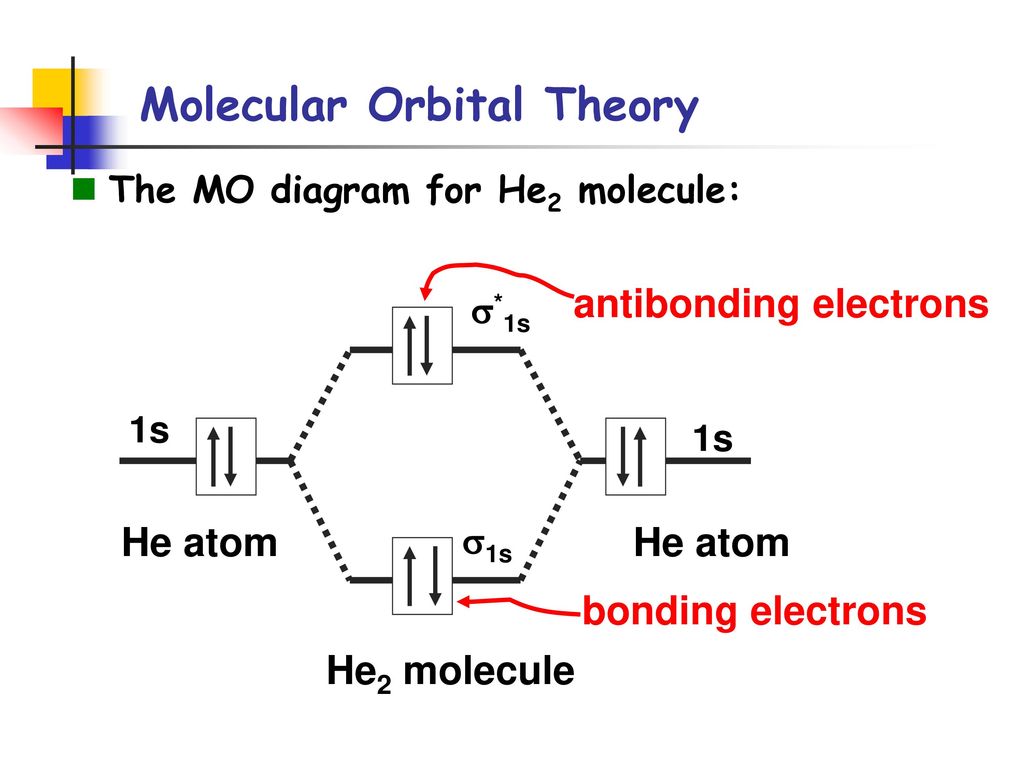
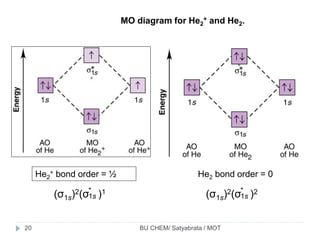
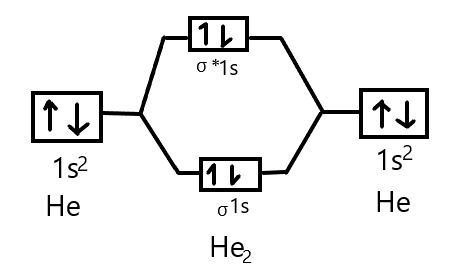
Molecular Orbital Diagram For He2+ - schematron.org The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule. Two are placed in the bonding orbital, the other two in antibonding orbital. The bond order = 1/2 x (Number of Bonding Electrons - Number of Antibonding Electrons) = .

Molecular orbital diagram for he2
Why He2 molecule does not exist? Explain by MOT. Solution Verified by Toppr Electronic configuration of He is 1s 2. Molecular Orbital Diagram for He 2 is (Refer to Image) Bond order= 2(No. of electrons in bonding molecular orbital)- (No. of electrons in anti-bonding Molecular orbital) = 22−2 =0 ∴He 2 bond order is 0. There is no bond existing between atoms of He 2 . So He 2 does not exist. How to Make the Molecular Orbital Diagram for He2: Does ... The bond order of He2 is calculated and the meaning of this number ... This video discusses how to draw the molecular orbital (MO) diagram for the He2 molecule. Mo Diagram He2 The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H 2 molecule is shown in Figure On either side of the central ladder are shown the energies of the 1 s orbitals of atoms A and B, and the central two-rung ladder shows the energies of the bonding and antibonding.
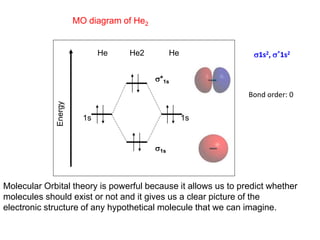
Molecular orbital diagram for he2. Do He2, He2(+), He2(2+) exist, stable? (Molecular Orbital ... In He2 (dihelium), the two 1s atomic orbitals overlap to create two molecular orbitals: sigma(1s) and sigma(1s)*. You fill these molecular orbitals with the... Molecular Orbital Diagram For He2 - schematron.org A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+.The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule. EOF Molecular Orbital Diagram For He2 A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [(2 − 2)/2 = 0], and we can make H+. According to the molecular orbital theory, in a supposed He2 molecule, both the if we draw its MOT DIAGRAM, 2 e's enter the Bonding molecular Orbital and 2 .
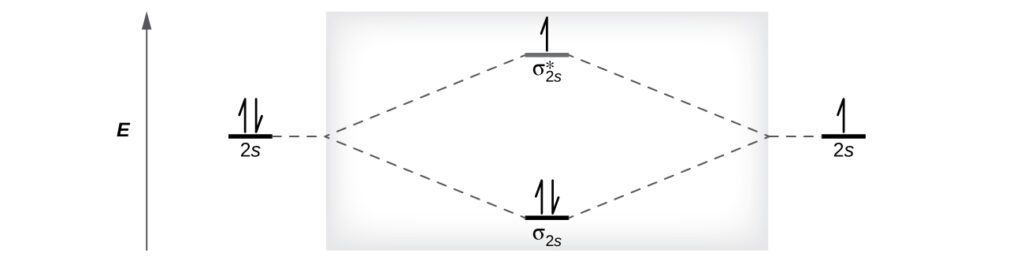
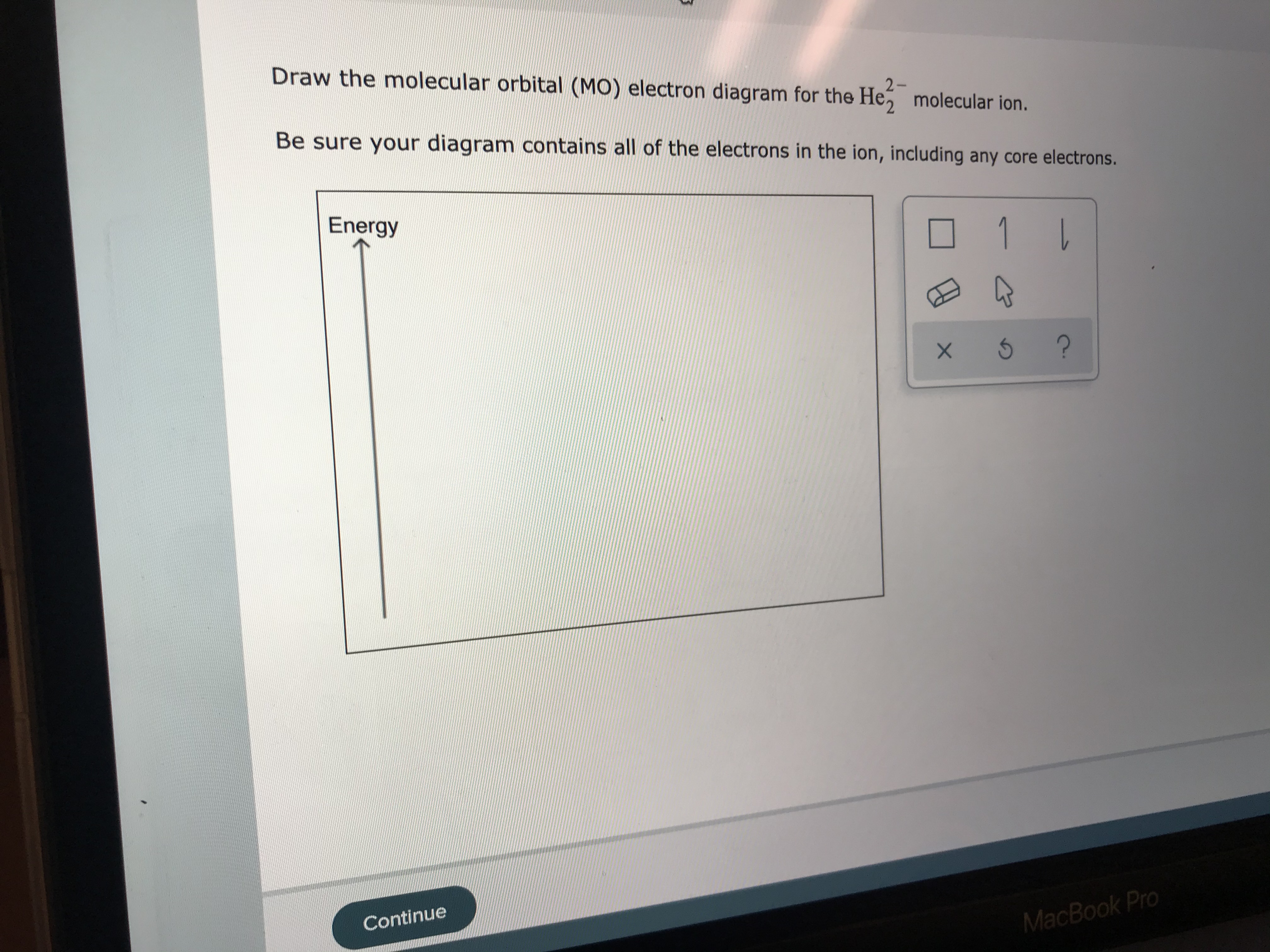
He2 2+ Molecular Orbital Diagram - Wiring Diagrams Please note the diagram is for He2+ but the He-H is very similar. He2+ MO diagram. Eg: Li + H; Li has 1s + 2s, while H has 1s. A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [(2 − 2)/2 = 0], and we can make H+. 2,. H−. Bond order = 1. chemical bonding - Molecular orbitals of H2 and He2 ... The molecular orbital energy-level diagram shown in Figure 13 also applies (with changes of detail in the energies of the molecular orbitals) to the hypothetical species He 2. However, this species has four valence electrons, and its configuration would be 1σ 2 2σ 2. Solved Fill in the Molecular Orbital Energy Diagram for ... 98% (42 ratings) If you still have a …. View the full answer. Transcribed image text: Fill in the Molecular Orbital Energy Diagram for the diatomic molecule He2. heliump heliumA MO's * σ2s 2s * σ1s 1s Fill in the Molecular Orbital Energy Diagram for the diatomic molecule C2 carbong carbonA MO's 2p 2s σ2s * σ1s 1s 1s. Molecular Orbital Diagram He2 - wiringall.com He2 is not possible. He MO Diagram. Eg: He + H; same mixing as above. Three electrons, two in sigma, one in sigma*. One more electron in. Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe. Even rather simple molecular orbital (MO) theory can be used to predict which from the bottom of the diagram because this is how MO diagrams are constructed , MOs are more antibonding than bonding MOs are bonding, He2 (dihelium).
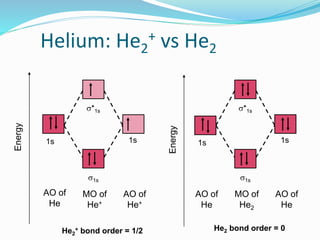
What is the bond order of He2? - Quora In He2 molecule, Atomic orbitals available for making Molecular Orbitals are 1s from each Helium. And total number of electrons available are 4. Molecular Orbitals thus formed are:€1s2€*1s2. It means 2 electrons are in bonding molecular orbitals and 2 are in antibonding molecular orbitals . Molecular orbital correlation diagrams for He2, He2+, N2 ... The correlation diagrams for nitrogen and carbon monoxide and the first positive ions of each symmetry type are all similar in shape, especially for the innermost orbitals. Ionization potential curves plotted on the diagrams for the neutral systems are nearly parallel to the corresponding orbital energy curves. Why is He2 not a stable molecule? - CHEMISTRY COMMUNITY Postby Chem_Mod » Wed Oct 26, 2016 6:31 am To answer the question, you must construct a molecular orbital (MO) diagram for the hypothetical He 2 molecule. The σ 1s bonding and antibonding orbitals will be full. Calculating the bond order results in 0. In other words, no bond can be sustained between two He atoms according to MO theory. Top How do I calculate the bond order for H2- and H2+? | Socratic Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, "H"_2^(-) has three electrons while "H"_2^(+) has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one sigma_(1s) and one sigma_(1s)^"*" MO by conservation of orbitals.
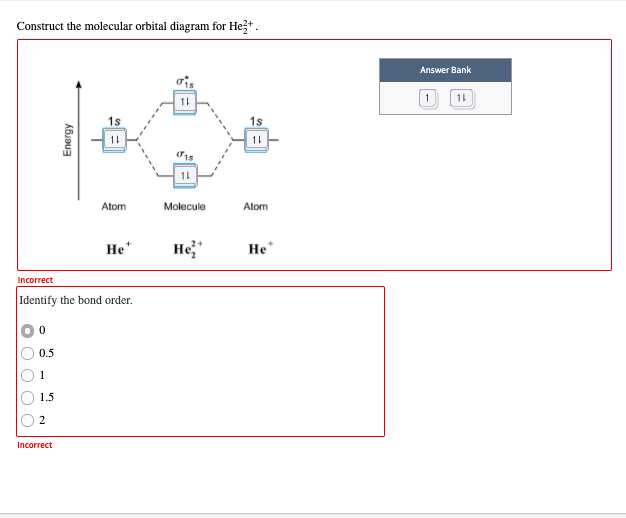
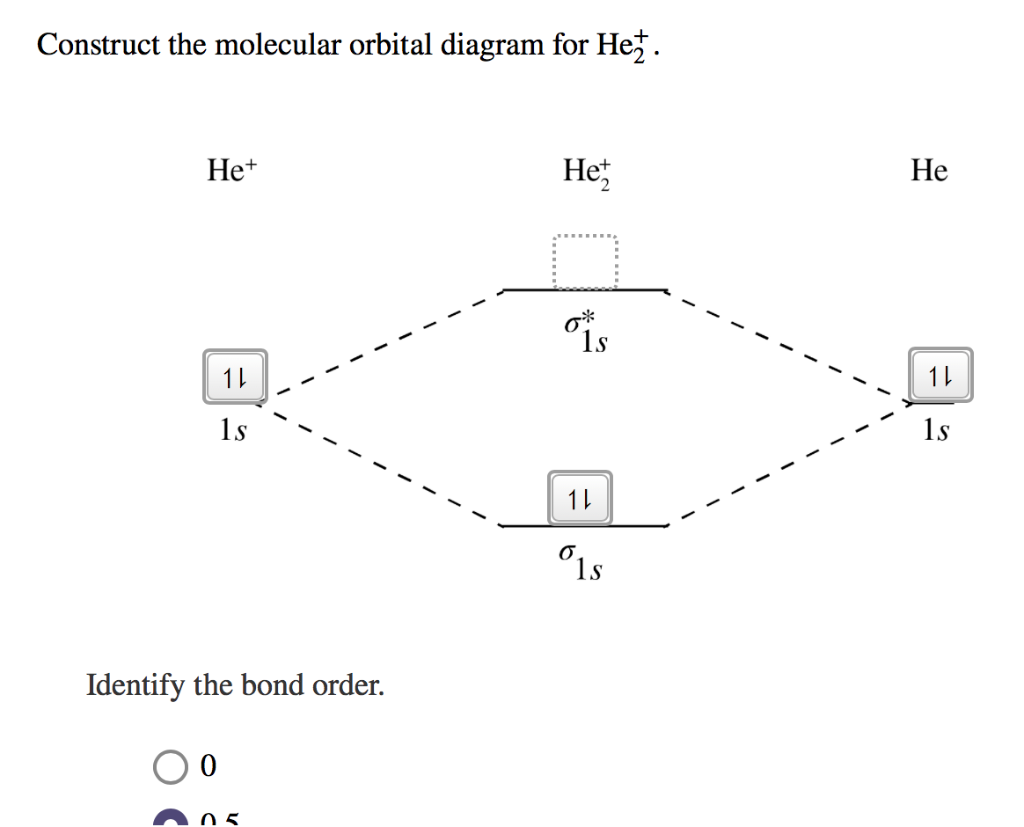
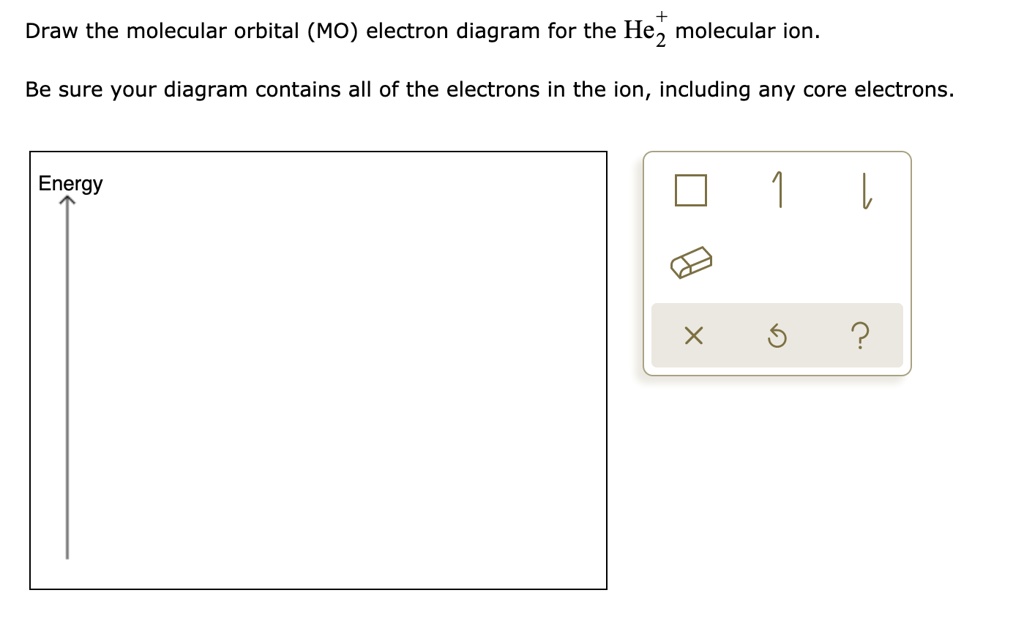
Solved Draw the molecular orbital diagram for He2 + . Drag ... Transcribed image text: Draw the molecular orbital diagram for Hez Drag the appropriate labels to their respective targets. Reset th Atomic orbital Molecular orbitals Atomic orbital 11 ॥ Antibonding ls ls Energy # He atom Bonding Het ion 1+1 11 He2+ ion
Why does He2 not exist? - Quora The reason oh He2 Molecule to not exist can be explained on the basis of. 1)MOLECULAR ORBITAL THEORY . He has configuration of 1s2 , if we draw its MOT DIAGRAM , 2 e's enter the Bonding molecular Orbital and 2 e's enter the AntiBonding molecular Orbital , thus net effect of the anti bonding and bonding is cancelled .
Mo Diagram He2 The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H 2 molecule is shown in Figure On either side of the central ladder are shown the energies of the 1 s orbitals of atoms A and B, and the central two-rung ladder shows the energies of the bonding and antibonding.
How to Make the Molecular Orbital Diagram for He2: Does ... The bond order of He2 is calculated and the meaning of this number ... This video discusses how to draw the molecular orbital (MO) diagram for the He2 molecule.
Why He2 molecule does not exist? Explain by MOT. Solution Verified by Toppr Electronic configuration of He is 1s 2. Molecular Orbital Diagram for He 2 is (Refer to Image) Bond order= 2(No. of electrons in bonding molecular orbital)- (No. of electrons in anti-bonding Molecular orbital) = 22−2 =0 ∴He 2 bond order is 0. There is no bond existing between atoms of He 2 . So He 2 does not exist.
















0 Response to "38 molecular orbital diagram for he2"
Post a Comment