38 cs molecular orbital diagram
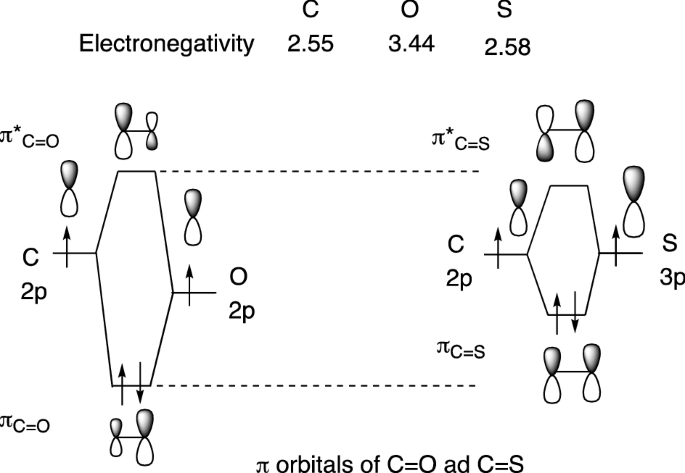
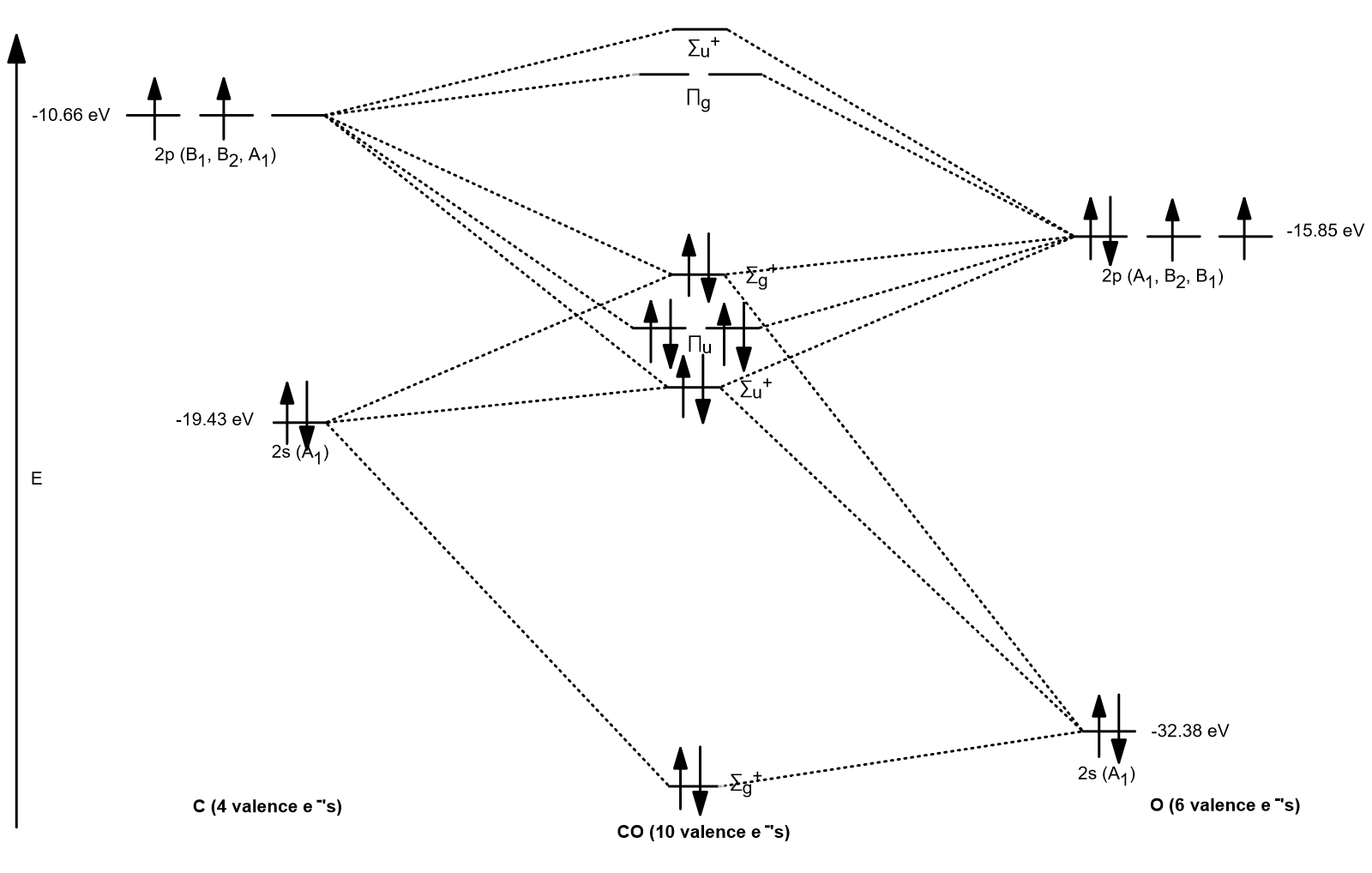
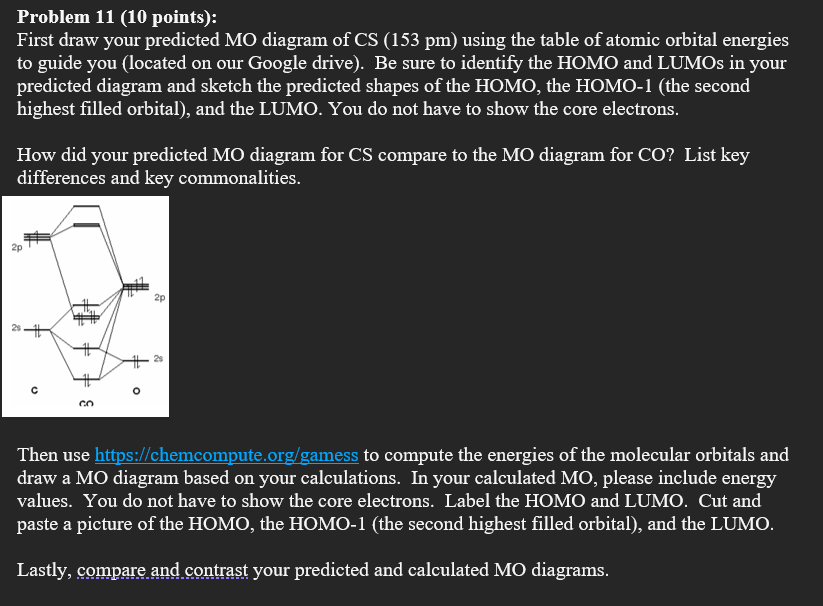
PDF Chem 344 - Homework 9 due Friday, Apr. 11, 2014, 2 PM orbitals are used. So CS is like C≡O except you use 3s,3p orbitals for . the example thus ignores 1s on C and 1s,2s,2p on S Similar point, Cl uses 3s,3p and Br ... P16.17) Sketch a molecular orbital energy diagram for CO and place the electrons in the levels appropriate for the ground state. The AO ionization energies are O2s: 32.3 eV; ... PDF Ground State Electron Configurations - University of Guelph As a result, the atomic orbitals contract (become less diffuse). The reduction in the "diffuse-ness" of the 4s orbital results in an increase in e-e repulsion between electrons occupying this orbital. For M+, this reduction in the "diffuse-ness" of the 4s orbital does not always
SOLVED:Draw the molecular orbital diagram of CS. Determine ... Draw the molecular orbital diagram of CS. Determine bond order, magnetism, spin multiplicity, and HOMO/LUMO. Colton B. Liberty University Answer Draw the molecular orbital diagram of the valence shell of a F 2 + ion, and use it to determine the bond order in the ion. Chemistry: An Atoms-Focused Approach Chapter 5
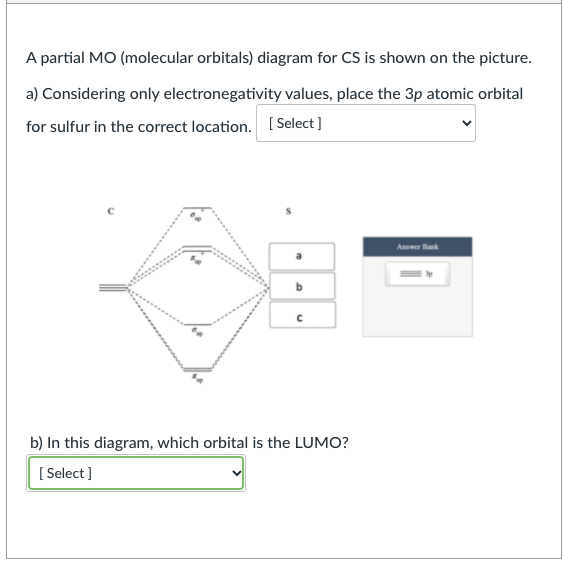
Cs molecular orbital diagram
4.9: Molecular Orbitals - Chemistry LibreTexts (a) This diagram shows the formation of a bonding σ1s molecular orbital for H2 as the sum of the wave functions (Ψ) of two H 1s atomic orbitals. (b) This plot of the square of the wave function (Ψ2) for the bonding σ1s molecular orbital illustrates the increased electron probability density between the two hydrogen nuclei. NHF2 Cs - ChemTube3D NHF 2 C s. 1 model in this collection. Use getProperty "modelInfo" or getProperty "auxiliaryInfo" to inspect them. Use "set autoLoadOrientation TRUE" before loading or "restore orientation DEFAULT" after loading to view this orientation. NHF 2 only contains a σ plane. What is the molecular orbital diagram for C2^ Maps Practical Geometry Separation of SubstancesPlaying With Numbers India: Climate, Vegetation and Wildlife. class 7. Inside Our Earth Perimeter and Area Winds, Storms and CyclonesStruggles for Equality The Triangle and Its Properties. class 8.
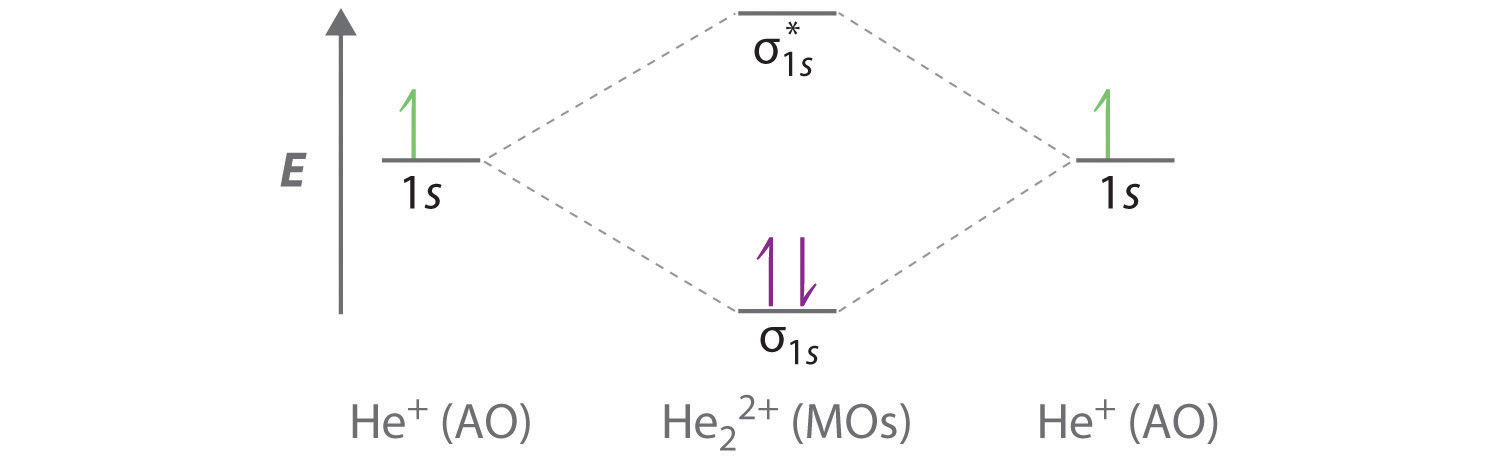
Cs molecular orbital diagram. Molecular Nitrogen and Related Diatomic Molecules When the electronegativity of one atom is lower than the other, the more electronegative atom's orbitals are lower in energy. The molecular orbital diagram of carbon monoxide, CO, is show below. On the left you can see all of the orbitals. On the right, the total valence electrons (4 from C, 6 from O) have been added to the orbitals. Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ... Carbon Disulfide (CS2) - Structure, Molecular Mass ... CS 2 is an organosulfur compound and a volatile liquid with chemical name Carbon Disulfide. It is also called Carbon bisulfide or disulfidocarbon or methanedithione. Carbon Disulfide is a solvent for sulfur, bromine, fats, rubber, phosphorus, asphalt, selenium, iodine, and resins. It has been widely used to purify single-walled carbon nanotubes ... 2.5.5: Molecular Orbital Diagrams - Chemistry LibreTexts Molecular Orbital Diagrams This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H2+. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic orbitals.
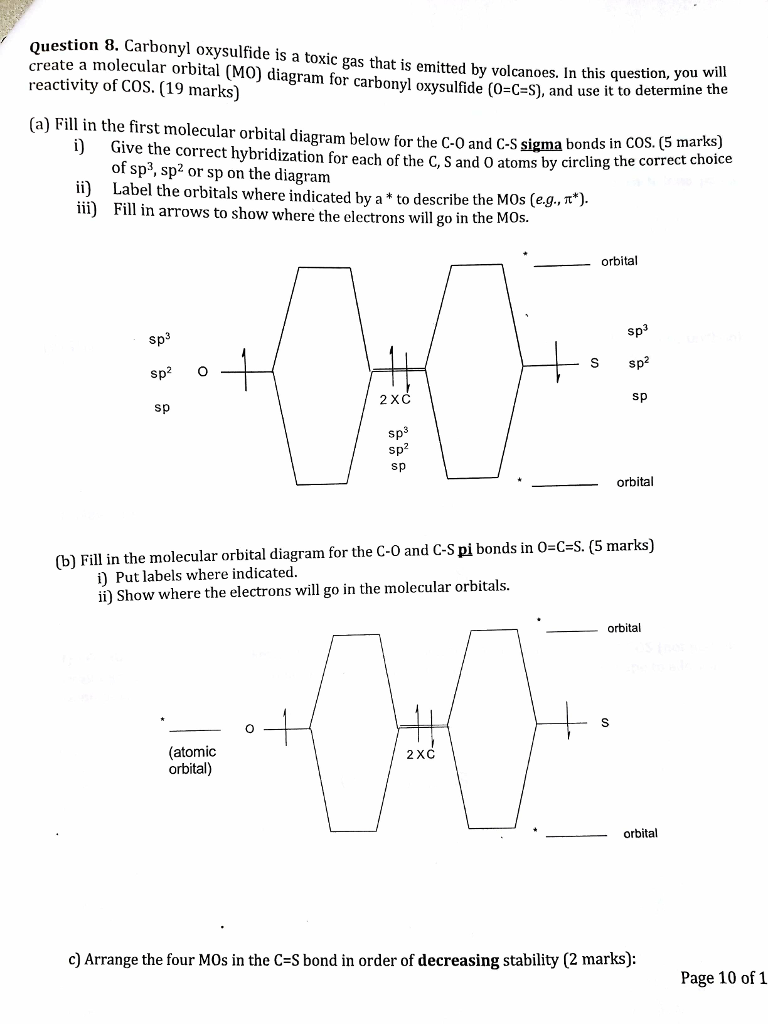
PDF Practice Test Questions 3 Molecular Orbital Theory ... (b) Develop the MO energy level diagram for 𝐿𝐿𝐿𝐿𝐿𝐿. Clearly indicate which atomic orbitals mix to make which molecular orbitals. Write the orbital occupancy for 𝐿𝐿𝐿𝐿𝐿𝐿, and estimate the bond order. (c) We would expect 𝐿𝐿𝐿𝐿𝐿𝐿 to have substantial ionic character. Does your MO work agree with PDF Miessler-Fischer-Tarr5e SM Ch 05 CM The populations of the bonding (8 electrons) and antibonding (4 electrons) molecular orbitals in the diagram suggest a double bond. c. The 2s, 2s *, 2p, and 2p *orbitals exhibit C vsymmetry, with the NF bond axis the infinite-fold rotation axis. The 2pand 2p * orbitals exhibit Cssymmetry. CS2 Lewis Structure, Hybridization, Molecular Shape, and ... Total valence electrons is CS2 = 12+4 = 16. 2. According to Step 2, carbon is the least electronegative having the highest bonding sites. C is the central atom here. 3. The skeleton diagram of CS2 is drawn below. 4. We can check and find out that both the sulfur atoms have fulfilled their octet rule here. Molecular Orbital Theory - Purdue University The two electrons associated with a pair of hydrogen atoms are placed in the lowest energy, or bonding, molecular orbital, as shown in the figure below. This diagram suggests that the energy of an H 2 molecule is lower than that of a pair of isolated atoms. As a result, the H 2 molecule is more stable than a pair of isolated atoms.
OneClass: How are N2, CN-, and CS alike? Mark all of the ... In the molecular orbital representation of benzene, how many pi-molecular orbitals are present? a) I b) 3 c)4 d)6 7. In the IR. w hat wave number in the spectrum is for the C-H stretching observed in the aromatic ring? a) 3300cm'1 b) 3030cm1 c)2950cm'' d) 2300cm'1 8. Which of the following is also an acceptable name for 2-nitroanisol? ChemEd DL Application: Models 360 Find: Models 360. x. (June 2021) The site is temporarily down. This is a standalone version of Models360 and links to ChemEdDL will not work. Xavier Prat-Resina is administering the current version of Models360 and its development, contact him (pratr001@umn.edu) for any question. Molecules Solids. CS2 Molecular Geometry - Science Education and Tutorials The first step is to sketch the molecular geometry of the CS2 molecule, to calculate the lone pairs of the electrons in the central carbon and terminal sulfur atoms; the second step is to calculate the CS2 hybridization, and the third step is to give perfect notation for the CS2 molecular geometry. What is the molecular geometry of the CS2 ... - Socratic.org Explanation: The best place to start when trying to figure out a molecule's geometry is its Lewis structure. Carbon disulfide, CS2, will have a total of 16 valence electrons, 4 from the carbon atom and 6 from each of the two sulfur atoms. The central carbon atom will form double bonds with the two sulfur atoms.
Molecular Orbital Theory - GeeksforGeeks The Molecular Orbital Theory is a chemical bonding theory developed at the turn of the twentieth century by F. R. Hund and R. S. Mulliken to explain the structure and properties of various molecules. The valence-bond theory failed to adequately explain how certain molecules, such as resonance-stabilized molecules, contain two or more equivalent ...
CS2 Lewis Structure, Hybridization, Polarity and Molecular ... In CS2 molecule, two double bonds are formed consisting of eight valence electrons. Thus it takes up eight valence electrons out of 16 valence electrons. These valence electrons that form the double bond with the Carbon atom are in 2s and 2p orbital of the Carbon atom. These orbitals then combine, creating the hybrid of sp orbitals.
Atomic Orbital Diagram of a Neutral Atom of Cesium - YouTube Is this the correct atomic orbital diagram for a neutral atom of cesium (Cs)? Please indicate "true" or "false" accordingly on your Google quiz form.
Solved How many unpaired electrons are there when ... - Chegg The molecular orbitals; Question: How many unpaired electrons are there when 1 Carbon and 1 Sulfur atom combine to form (CS)? and DRAW the molecular orbital diagram for it, showing all the details of each orbital. How many unpaired electrons are there in CN- and DRAW the molecular orbital diagram for it, showing all the details of each orbital.
Solved Question 21 of 25 One of the molecular orbitals of ... Answer is option (A) Explanation:- Since the molecular orbital in the picture is formed along the internuclear axis of the m …. View the full answer. Transcribed image text: Question 21 of 25 One of the molecular orbitals of CS (SEC=S) is shown below. Identify the type of orbital. A) B) C) TT D) TT E) nonbonding.
the Cs point group - LMU The C s Point Group. The C. s. Point Group. This point group contains only two symmetry operations: E the identity operation. σ a mirror plane. A simple example for a C s symmetric molecule is methanol (CH 3 OH) in its staggered conformation, here in its HF/6-31G (d) optimized structure: #P HF/6-31G (d) opt= (Z-Matrix,tight) test1 HF/6-31G (d ...
What is the molecular orbital diagram for C_2^-? | Socratic As you can see in the diagram, the two 2pπ orbitals, let's say 2pπx and 2pπy, are the highest energy occupied molecular orbitals. The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram
PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
How To Find an Valence Cesium Electron Configuration (Cs) Every element has its own Cesium Electron Configuration whereas here the electronic configuration for the element Cs is written as [Xe] 6 s1. The melting point of caesium is 83.3 o F and if we convert it to Celsius form, then it will be written as 28.5o C among the five elemental metals, cesium is one of the elements which are at room temperature.
CN Lewis Structure, Molecular Geometry, Hybridization ... Molecular Orbital Diagram. Molecular Orbital Theory is slightly different from VBT and orbital hybridization. Here, AOs from different atoms inside the molecule can come together to form molecular orbitals or MOs. Therefore, valence electrons are shared inside the molecule. The electronic configuration of both C and N are as follows: Carbon ...
What is the molecular orbital diagram for C2^ Maps Practical Geometry Separation of SubstancesPlaying With Numbers India: Climate, Vegetation and Wildlife. class 7. Inside Our Earth Perimeter and Area Winds, Storms and CyclonesStruggles for Equality The Triangle and Its Properties. class 8.
NHF2 Cs - ChemTube3D NHF 2 C s. 1 model in this collection. Use getProperty "modelInfo" or getProperty "auxiliaryInfo" to inspect them. Use "set autoLoadOrientation TRUE" before loading or "restore orientation DEFAULT" after loading to view this orientation. NHF 2 only contains a σ plane.
4.9: Molecular Orbitals - Chemistry LibreTexts (a) This diagram shows the formation of a bonding σ1s molecular orbital for H2 as the sum of the wave functions (Ψ) of two H 1s atomic orbitals. (b) This plot of the square of the wave function (Ψ2) for the bonding σ1s molecular orbital illustrates the increased electron probability density between the two hydrogen nuclei.








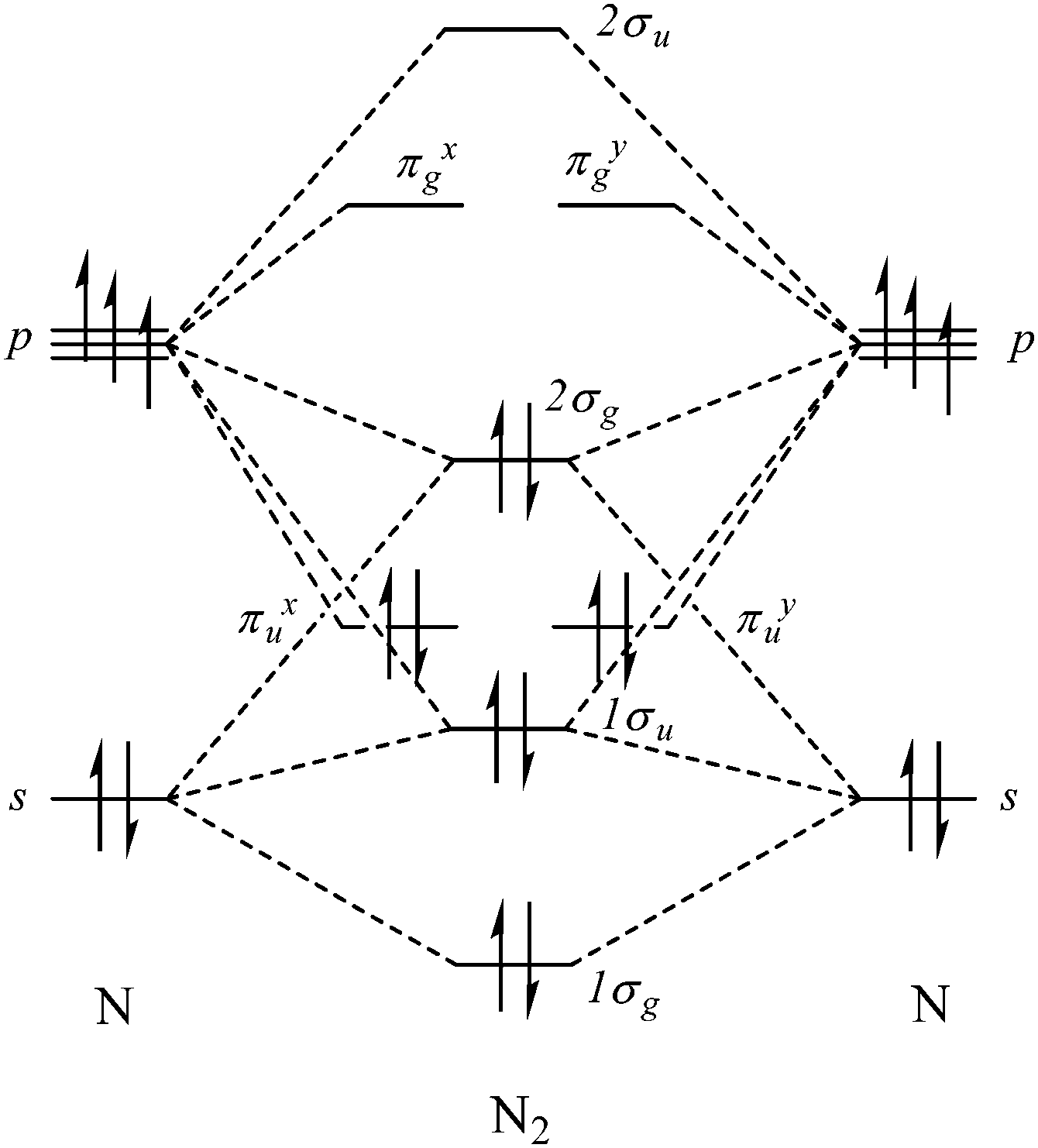




0 Response to "38 cs molecular orbital diagram"
Post a Comment