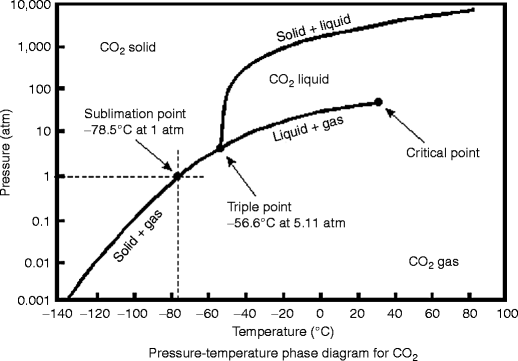
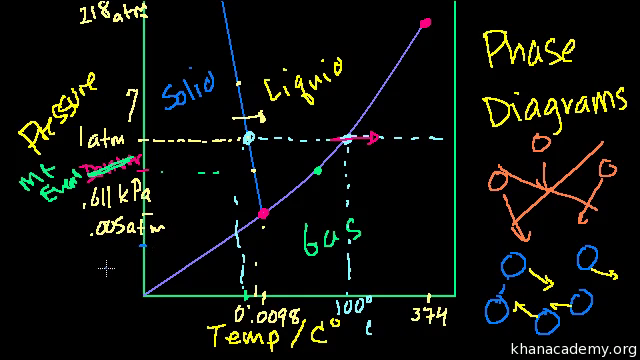
42 consider this phase diagram for carbon dioxide.
Carbon Dioxide Phase Diagram Wikipedia - 16 images ... [Carbon Dioxide Phase Diagram Wikipedia] - 16 images - 35 consider this phase diagram for carbon dioxide, solved referring to the phase diagram for carbon dioxide, phase diagram carbon dioxide 002 youtube, critical state of carbon dioxide collection of experiments, Solved Consider this phase diagram for carbon dioxide, CO_2 ... Consider this phase diagram for carbon dioxide, CO_2, below. In what is CO_2 at 4 bar and -10^degree C? solid liquid gas Starting from the point described above, what phase change would eventually result from an increase in pressure? freezing vaporization deposition sublimation melting. Question: Consider this phase diagram for carbon dioxide ...
Label The Phase Diagram For Carbon Dioxide - 18 images ... [Label The Phase Diagram For Carbon Dioxide] - 18 images - ppt warm up powerpoint presentation id 2476886, phase diagrams, phase diagram carbon dioxide alyce flickr, dense phase carbon dioxide food and pharmaceutical,

Consider this phase diagram for carbon dioxide.
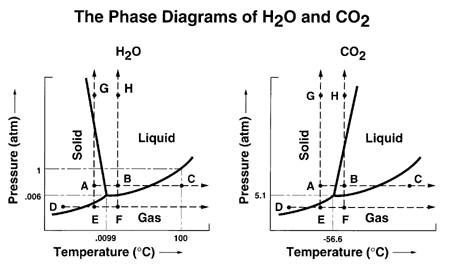
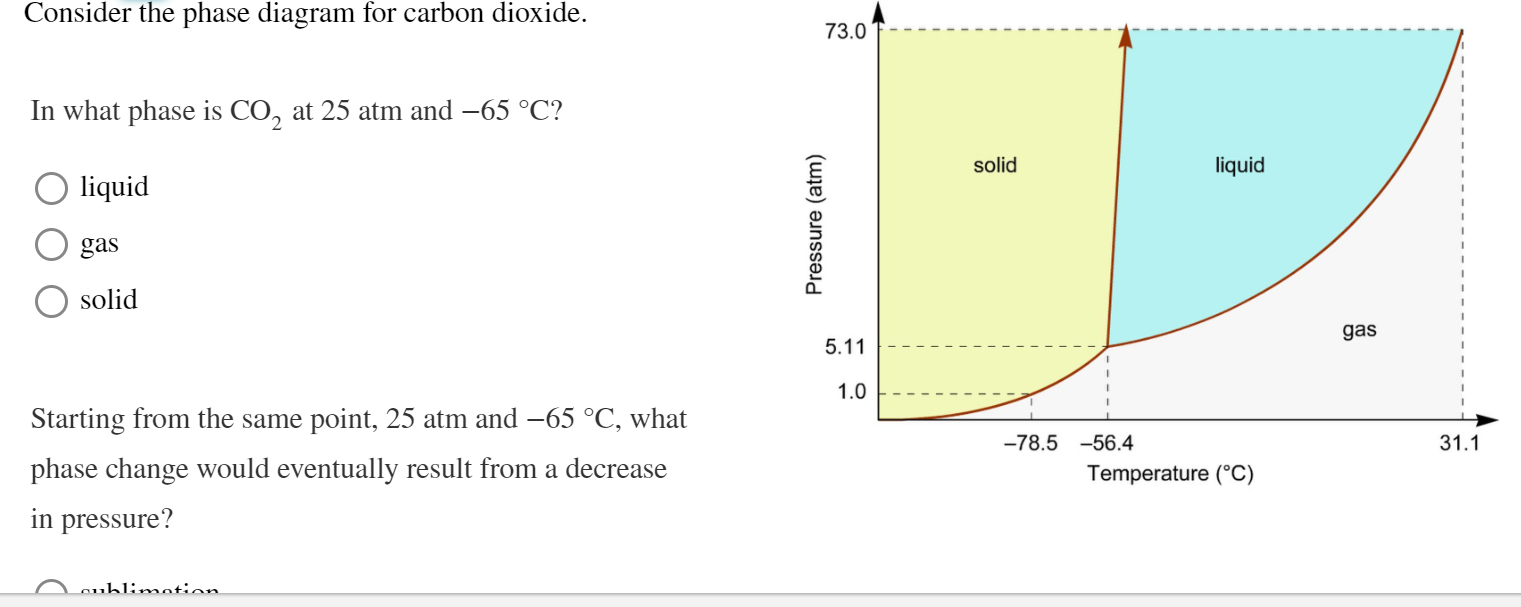
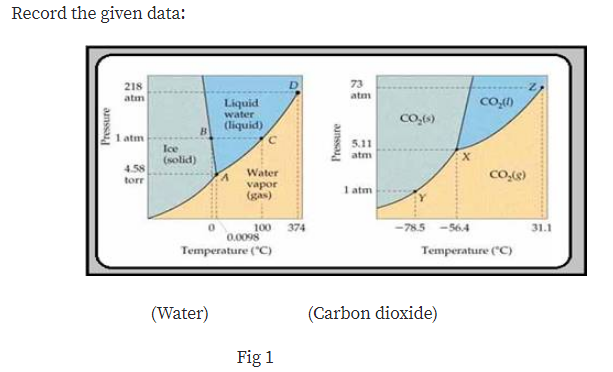
Answered: Consider the phase diagram for carbon… | bartleby Consider the phase diagram for carbon dioxide. 73.0 In what phase is CO, at 25 atm and -65 °C? solid liquid liquid gas solid gas 5.11 1.0 3D -78.5 -56.4 31.1 Temperature (°C) Starting from the same point, 25 atm and -65 °C, what phase change would eventually result from a decrease in pressure? freezing sublimation melting vaporization condensation deposition Pressure (atm) Consider the phase diagram for carbon diox... | Clutch Prep Problem Details. Consider the phase diagram for carbon dioxide. In what phase is CO2 at 4 atm and -10 °C? a) liquid b) gas c) solid Starting from the same point, 4 atm and -10 °C, what phase change would eventually result from an increase in pressure? a) sublimation b) melting c) freezing d) condensation e) vaporization f) deposition. Consider the phase diagram of carbon dioxide below Which ... Consider the phase diagram of carbon dioxide below: Which term best describes the process that occurs in going from point D to point C? a) vaporization b) condensation c) sublimation d) deposition e) fusion. Point D is in gas phase and point C is in solid phase. The transition of substance from solid to gas directly is termed as sublimation. 10.
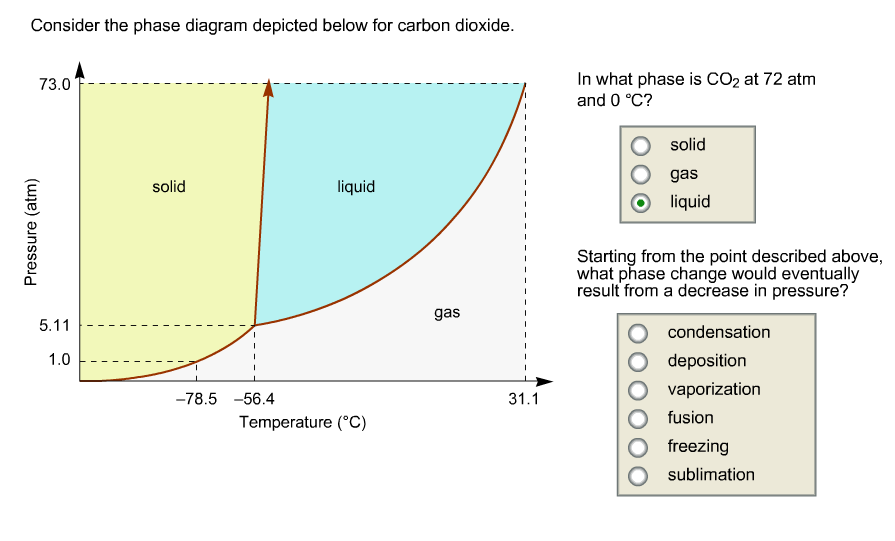
Consider this phase diagram for carbon dioxide.. Solved Consider this phase diagram for carbon dioxide, CO2 ... Consider this phase diagram for carbon dioxide, CO2. 730 In what phase is CO2 at 4 bar and -10°C? Solid Liquid liquid Pressure (bar) gas solid Gas 5.11 10 31.1 78.5564 Temperature (°C) Starting from 4 bar and -10°C, what phase change would eventually result from an increase in pressure? deposition melting condensation sublimation O vaporization Adobe Scan Feb 22, 2021 (9).pdf - Consider this phase ... View Adobe Scan Feb 22, 2021 (9).pdf from CHM 112 at Mercer University. Consider this phase diagram for carbon dioxide. Phase changes in carbon dioxide 10,000 1,000 Liquid S Solved Consider this phase diagram for carbon dioxide. In ... Consider this phase diagram for carbon dioxide. In what phase is CO2 at 72 atm and 0 degree C? Starting from the point described above, what phase change would eventually result from a decrease in pressure? Question: Consider this phase diagram for carbon dioxide. In what phase is CO2 at 72 atm and 0 degree C? Answered: Consider the phase diagram for carbon… | bartleby Consider the phase diagram for carbon dioxide. 73.0F In what phase is CO, at 72 atm and 0 °C? solld iquid liquid solid gas 5.11 gas 1.0 Incorrect -78.5 -56.4 31.1 Temperature ("C) Starting from the same point, 72 atm and 0 °C, what phase change would eventually result from a decrease in pressure? sublimation freezing melting deposition vaporization condensation Pressure (atm)
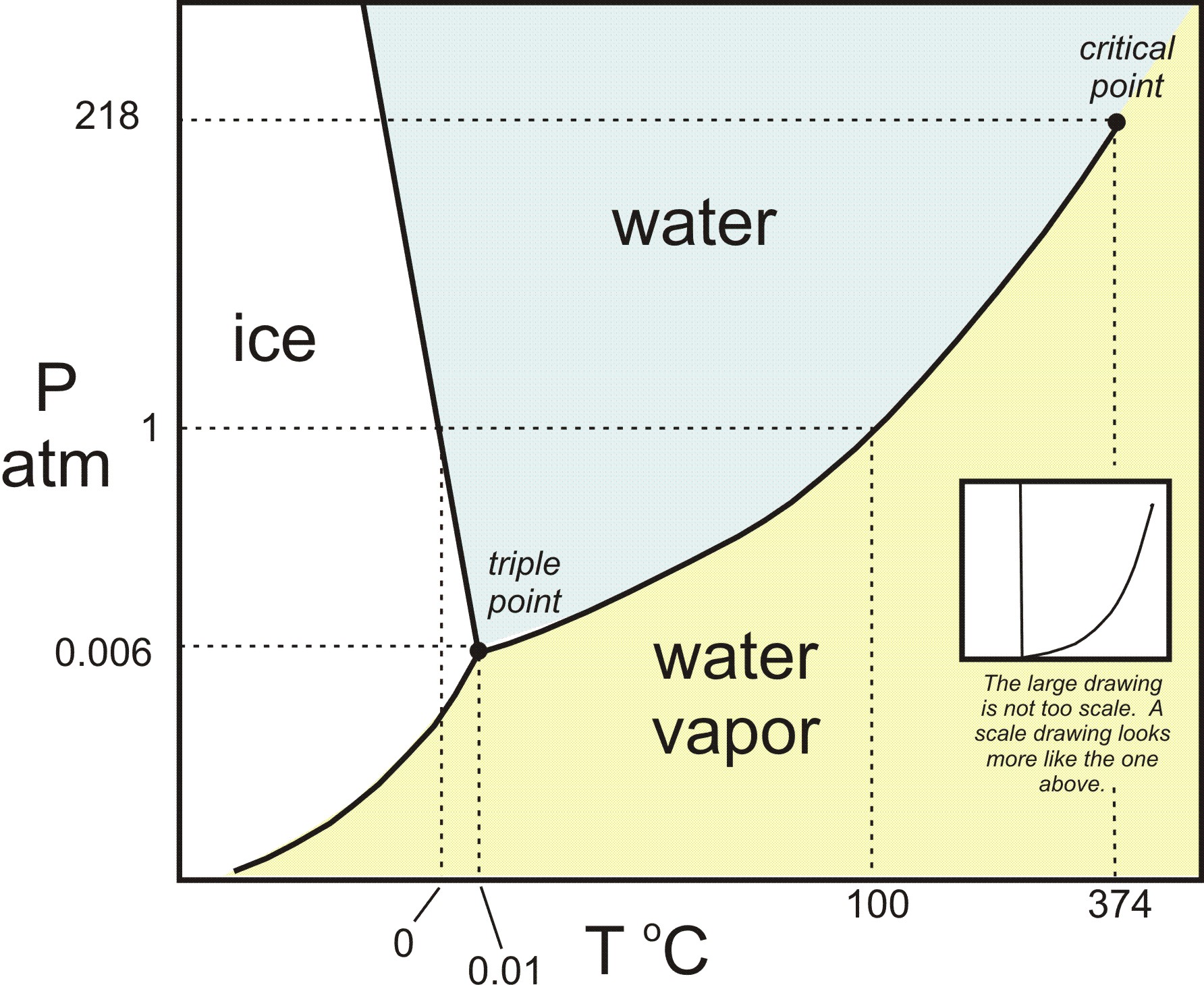
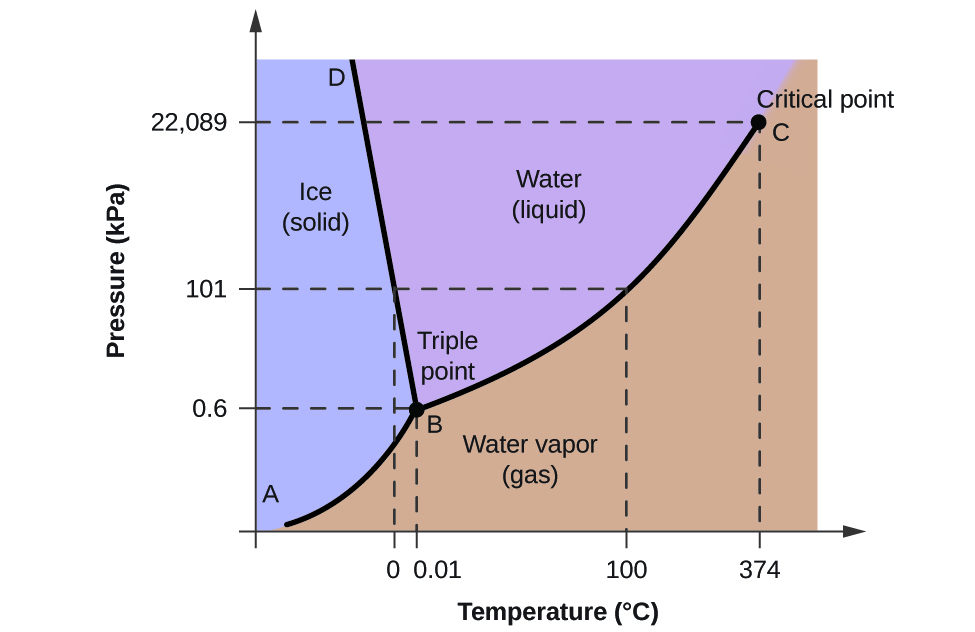
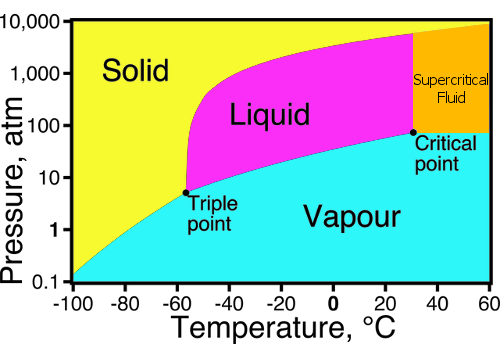
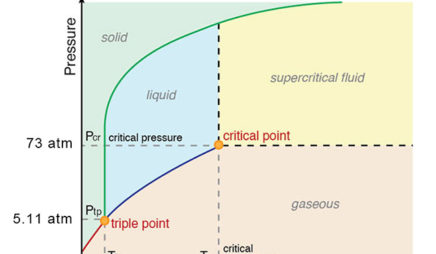
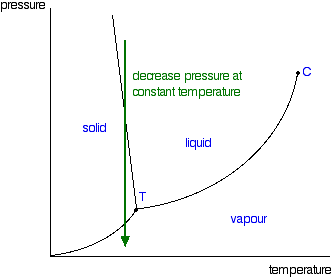
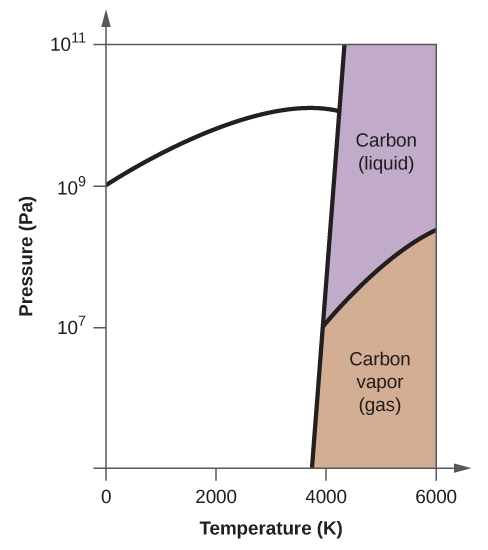
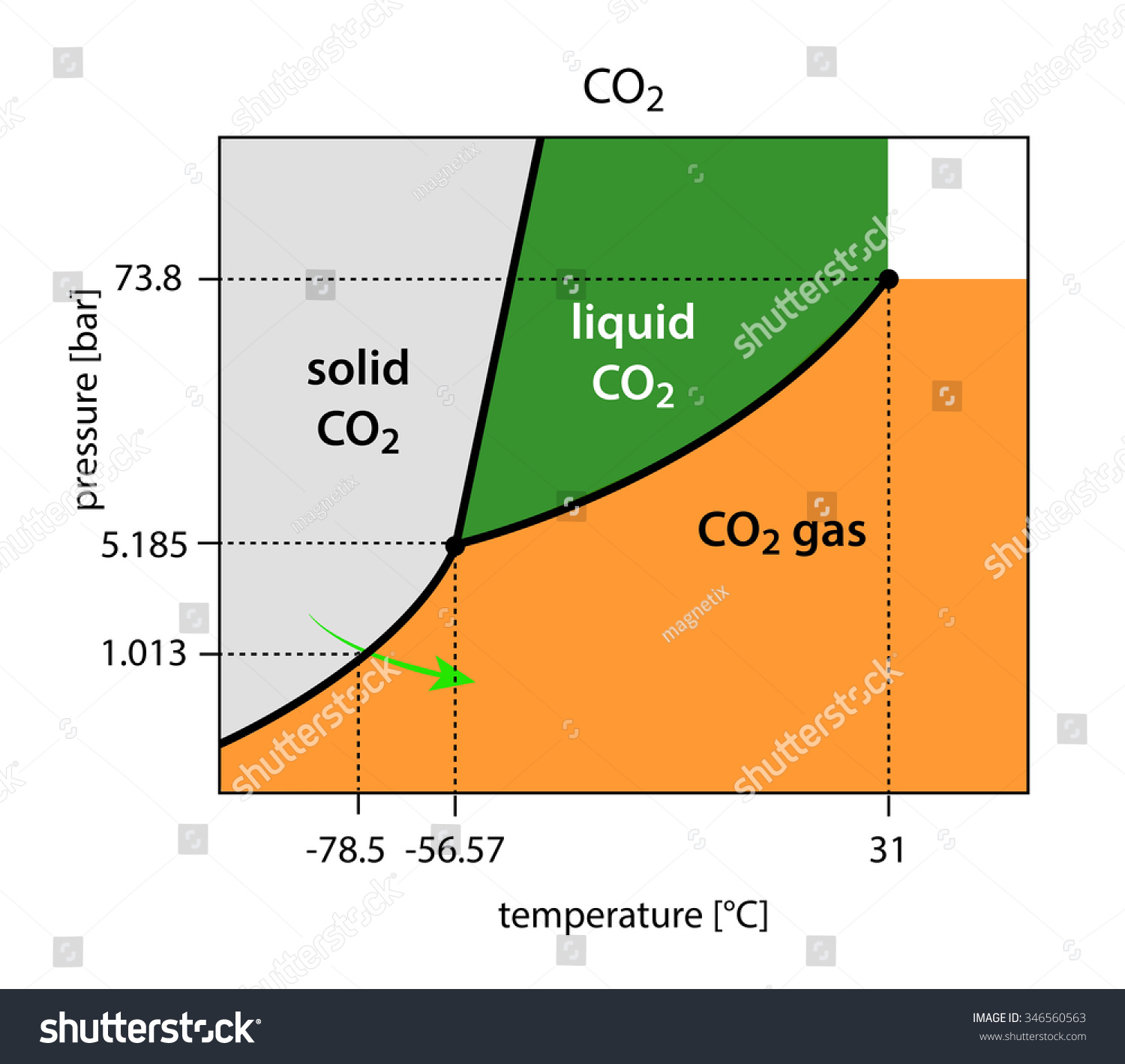
Consider the phase diagram for carbon diox... - Organic ... Consider the phase diagram for carbon dioxide, 73.0 In what phase is Co, at 72 atm and 0 °C? solid liquid solid Pressure (atm) gas liquid 5.11 gas 1.0 -78,5 -56.4 Temperature ("C) 31.1 Starting from the same point, 72 atm and 0 °C, what phase change would eventually result from a decrease in pressure? Gallery of 29 consider this phase diagram for carbon dioxide ... Mar 24, 2022 · 29 Consider This Phase Diagram For Carbon Dioxide Wiring images that posted in this website was uploaded by Sgi.gene.com.gene.com. 29 Consider This Phase Diagram For Carbon Dioxide Wiring equipped with a HD resolution 720 x 540.You can save 29 Consider This Phase Diagram For Carbon Dioxide Wiring for free to your devices. Phase Diagrams: Carbon Dioxide and Water Phase Diagrams ... Consider the phase diagram for carbon dioxide as another example. Figure 3. Phase diagram of carbon dioxide. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances. Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot ... OneClass: Compare and contrast the phase diagrams of water ... Compare and contrast the phase diagrams of water and carbon dioxide. a) why doesn't CO 2 have a normal boiling point and normal melting point, whereas water does?. b) The slopes of solid-liquid lines in the phase diagram of H 2 O and CO 2 are different. What do the slopes of solid-liquid lines indicate in terms of the relative densities of the solid and liquid states for each substance?
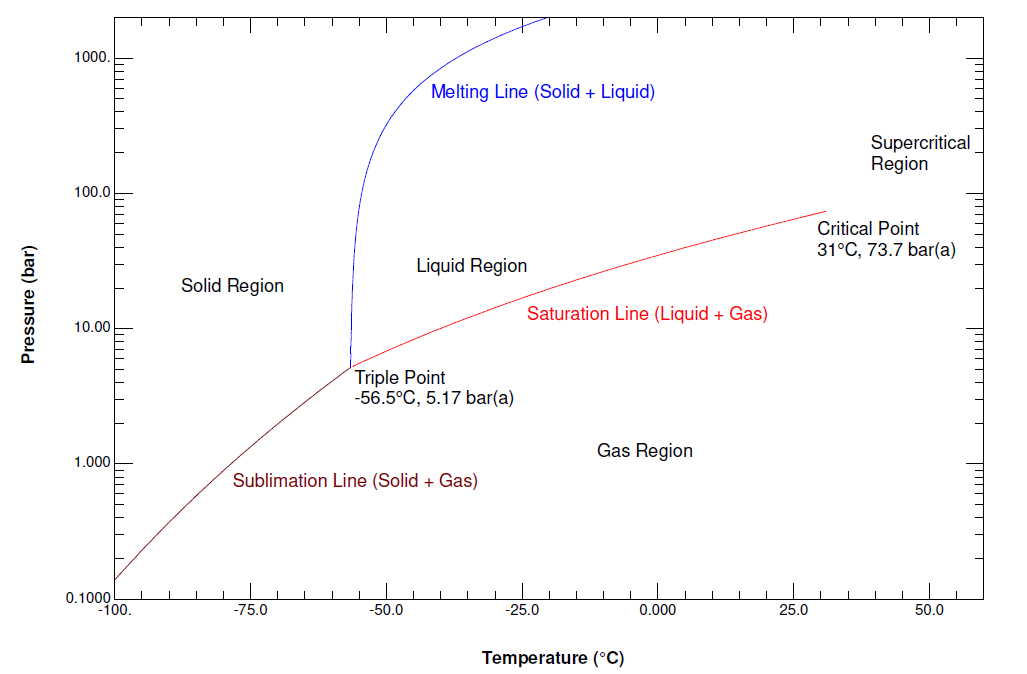
Gallery of phase diagram for carbon dioxide free wiring ... Phase Diagram For Carbon Dioxide Free Wiring Diagram images that posted in this website was uploaded by Sgi.gene.com.gene.com. Phase Diagram For Carbon Dioxide Free Wiring Diagram equipped with a HD resolution 480 x 360.You can save Phase Diagram For Carbon Dioxide Free Wiring Diagram for free to your devices. Consider this phase diagram for carbon dioxide. In what phas Consider this phase diagram for carbon dioxide. In what phase is CO2 at 72 atm and 0 degree C? Start Phase Diagrams - Chemistry - University of Hawaiʻi Consider the phase diagram for carbon dioxide shown in as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating ... phase diagrams of pure substances - chemguide The phase diagram for carbon dioxide. The only thing special about this phase diagram is the position of the triple point which is well above atmospheric pressure. It is impossible to get any liquid carbon dioxide at pressures less than 5.11 atmospheres. That means that at 1 atmosphere pressure, carbon dioxide will sublime at a temperature of ...
OneClass: Consider this phase diagram for carbon dioxide ... Consider this phase diagram for carbon dioxide. In what phase is CO_2 at 72 atm and 0 degree C? solid gas liquid Starting from the point described above, what phase change would eventually result from a decrease in pressure? condensation deposition sublimation freezing vaporization melting
differentiate the phase diagram of water from carbon dioxide Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. gaseous carbon dioxide explanation: The point on the phase diagram that represents the intersection of 300K and 10 bar lies squarely in the region labeled "gas." I find that satisfyingly bizarre! The table below gives thermodynamic data of liquid CO 2 in ...
(Get Answer) - Label the phase diagram for carbon dioxide ... consider this phase diagram for carbon dioxide Consider this phase diagram for carbon dioxide. In what phase is CO_2 at 25 atm and -65 degree C? solid gas liquid Starting from the point described above, what phase change would eventually result from...
How Do The Phase Diagram For Water And Carbon Dioxide ... Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm ...
Solved Consider the phase diagram for carbon dioxide. In ... Consider the phase diagram for carbon dioxide. In what phase is CO2 at 25 atm and −65 °C? gas. solid. liquid. Starting from the same point, 25 atm and −65 °C, what phase change would eventually result from a decrease in pressure?
Solved Consider this phase diagram for carbon dioxide. In ... Consider this phase diagram for carbon dioxide. In what phase is CO_2 at 25 atm and -65 degree C? solid gas liquid Starting from the point described above, what phase change would eventually result from a decrease in pressure? freezing vaporization condensation deposition melting sublimation; Question: Consider this phase diagram for carbon ...
Consider this phase diagram for carbon dioxide. In what ... Consider this phase diagram for carbon dioxide. 73.0 In what phase is CO2 at 4 atm and -10°C? solid solid liquid liquid gas Starting from the point described in the first question, 5.11 gas what phase change would eventually result from an...
phase diagram triple point - Label The Phase Diagram For ... Read Or Download Gallery of phase diagram triple point - Label The Phase Diagram For Carbon Dioxide | nutrient cycles quiz, chemistry the central science chapter 11 section 6, phase diagram of carbon untpikapps, nodrama devops,
Consider this phase diagram for carbon dioxide 30T- In ... Hint Consider the phase diagram for carbon dioxide. 73.0 In what phase is CO, at 4 atm and -10 °C? solid liquid O gas Pressure (atm) solid liquid 5.11 1.0 31.1 -78.5-564 Temperature (°C) Starting from the same point, 4 atm and -10 °C, what phase change would eventually result from an increase in pressure? freezing deposition melting ...
Consider the phase diagram for carbon dio... - Physical ... Consider the phase diagram for carbon dioxide. 73.0 In what phase is Co, at 72 atm and 0 °C? solid liquid liquid Pressure (atm) gas O solid gas 5.11 1.0 31.1 -78.5 -56.4 Temperature (°C) Starting from the same point, 72 atm and 0 °C, what phase change would eventually result from a decrease in pressure?
OneClass: Consider this phase diagram for carbon dioxide. In ... Nov 28, 2020 · Consider this phase diagram for carbon dioxide. In what phase is CO 2 at 72 atm and 0°C?. a. solid. b. liquid. c. gas. Starting from the point described above, what phase change would eventually result from a decrease in pressure?
Consider the phase diagram of carbon dioxide below Which ... Consider the phase diagram of carbon dioxide below: Which term best describes the process that occurs in going from point D to point C? a) vaporization b) condensation c) sublimation d) deposition e) fusion. Point D is in gas phase and point C is in solid phase. The transition of substance from solid to gas directly is termed as sublimation. 10.
Consider the phase diagram for carbon diox... | Clutch Prep Problem Details. Consider the phase diagram for carbon dioxide. In what phase is CO2 at 4 atm and -10 °C? a) liquid b) gas c) solid Starting from the same point, 4 atm and -10 °C, what phase change would eventually result from an increase in pressure? a) sublimation b) melting c) freezing d) condensation e) vaporization f) deposition.
Answered: Consider the phase diagram for carbon… | bartleby Consider the phase diagram for carbon dioxide. 73.0 In what phase is CO, at 25 atm and -65 °C? solid liquid liquid gas solid gas 5.11 1.0 3D -78.5 -56.4 31.1 Temperature (°C) Starting from the same point, 25 atm and -65 °C, what phase change would eventually result from a decrease in pressure? freezing sublimation melting vaporization condensation deposition Pressure (atm)






:max_bytes(150000):strip_icc()/phase-changes-56a12ddd3df78cf772682e07.png)









0 Response to "42 consider this phase diagram for carbon dioxide."
Post a Comment