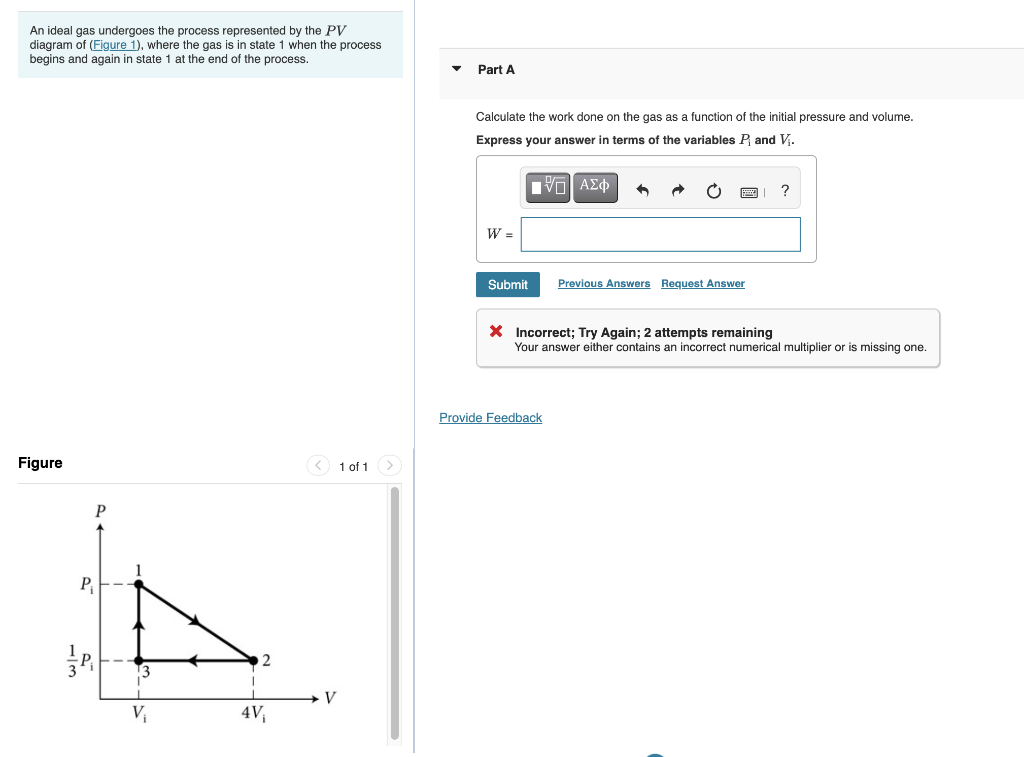
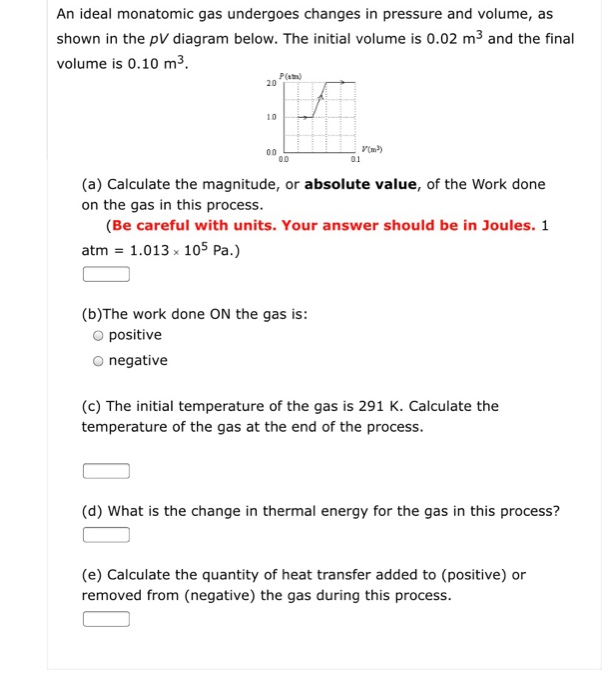
38 a gas undergoes a change of state described by the pvpv diagram shown in the figure below.
(PDF) Prediction of Phase Behavior of Spray-Dried ... Drugs and polymers are represented in the Bagl ey diagram, as illustrated in Figure 4. The The Euclidean distance was calculated between each poin t, and the results are provided in Table 4. Full text of "Fundamentals Of Classical Thermodynamics" Due to a planned power outage on Friday, 1/14, between 8am-1pm PST, some services may be impacted.
(PDF) Recent approaches of solid dispersion ... - ResearchGate Many solubilization techniques have been described that either change the nature of the solvent environment (cosol vent systems, emulsions, and micellization) or the chemical identity of the...

A gas undergoes a change of state described by the pvpv diagram shown in the figure below.
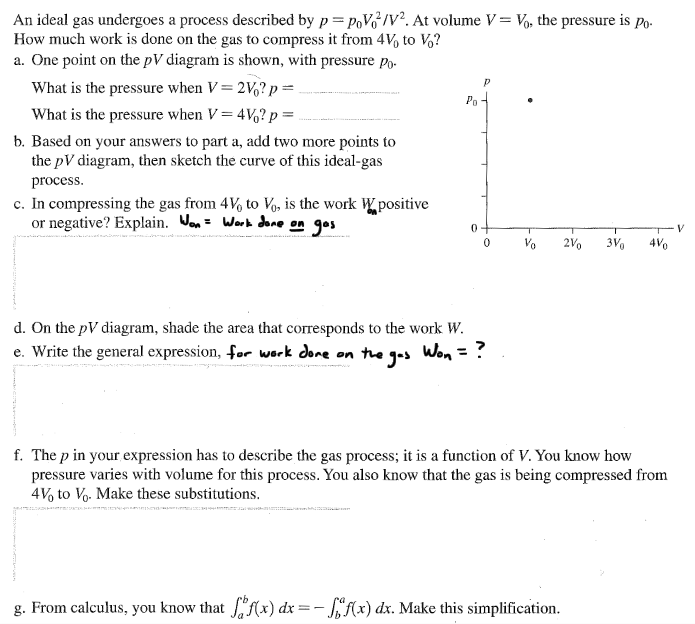
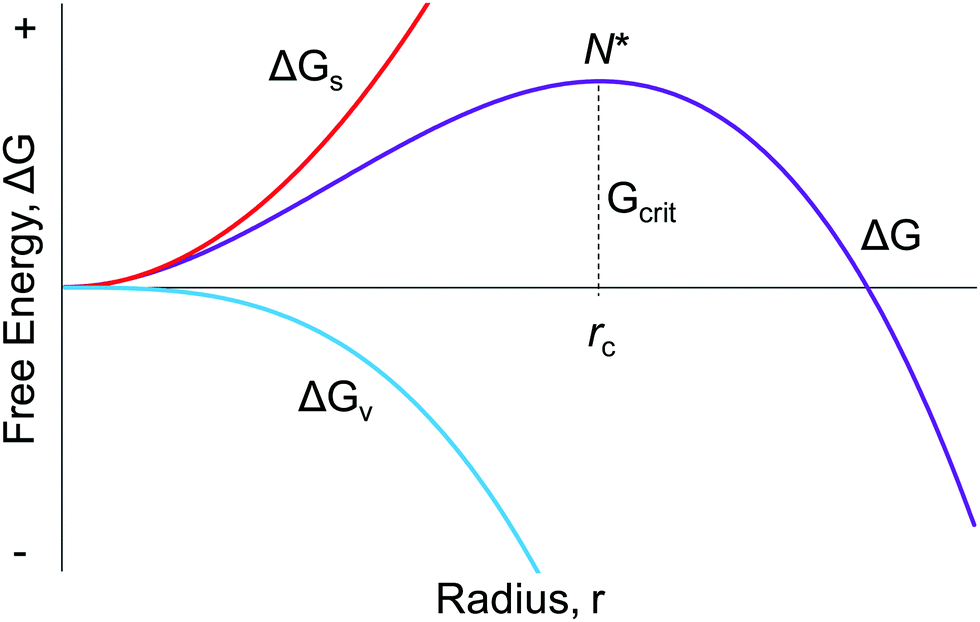
(PDF) The Influence of Drug Physical State on the ... Free energy-composition phase diagrams of OLZ and each polymer system (PVP, PVPVA, and SLP). +6 Differential scanning calorimetry curves of 50% drug-loaded formulations with SLP, PVPVA, and PVP... What are PV diagrams? (article) - Khan Academy Let's say our gas starts out in the state shown in the PV diagram below. If we press the piston downward, the volume of the gas will decrease, so the state must shift to the left toward smaller volumes (as seen in the diagram below). Since the gas is being compressed we can also say for sure that positive work is being done on the gas. Inhalational anaesthetics and n‐alcohols share a site of ... Several superimposed traces are shown before adding halothane, in the presence of 0.46 or 1 mM halothane and after washout (C, H and W respectively). (B) Bar graph summarizing the mean % change induced by two concentrations of halothane (0.46 or 1 mM). Negative and positive changes correspond to inhibition and potentiation respectively.
A gas undergoes a change of state described by the pvpv diagram shown in the figure below.. Full text of "JEE Main Chapterwise Explorer" An icon used to represent a menu that can be toggled by interacting with this icon. (PDF) Excipients That Facilitate Amorphous Drug Stabilization It is well known that the amorphous phase is thermodynamically unstable. This might result in the conversion of the metastable form to its stable crystalline form during storage. This conversion... (PDF) (Coulson & Richardson's Chemical ... - Academia.edu Academia.edu is a platform for academics to share research papers. Effects of Electron-Electron and Electron-Phonon ... The screening of the Coulomb interaction has a large influence on the dispersion relations of quasi particles, particularly in metals. We illustrate this with the case of an electron gas. As will be shown in Part VI, the energy of the quasi particle is given by Dyson's equation where Bk is the energy of a free particle a = fi2k2/2m. From Eq.
Inhalational anaesthetics and n-alcohols share a site of ... Several superimposed traces are shown before adding halothane, in the presence of 0.46 mM halothane and after washout (C, H and W respectively). The corresponding time-course of the experiment is shown below the traces. The horizontal black bar indicates the exposure of the oocyte to the indicated concentration of halothane. Solved A gas undergoes a change of state described by the ... Transcribed image text: A gas undergoes a change of state described by the pV diagram shown in the figure below. 4.00 3.00 2.00 1.00--- 0 0 1.00 2.00 3.00 4.00 V(m3) 1) Calculate the amount of work done on the gas. (Express your answer to three significant figures.) Inhalational anaesthetics and n-alcohols share a site of ... Several superimposed traces are shown before adding halothane, in the presence of 0.46 or 1 mM halothane and after washout (C, H and W respectively). (B) Bar graph summarizing the mean % change induced by two concentrations of halothane (0.46 or 1 mM). Negative and positive changes correspond to inhibition and potentiation respectively. Answered: Iridium crystallizes in a face-centered… | bartleby Science Chemistry Q&A Library Iridium crystallizes in a face-centered cubic unit cell that has an edge length of 3.833 Å. Calculate the atomic radius of an iridium atom. Calculate the density of iridium metal. Iridium crystallizes in a face-centered cubic unit cell that has an edge length of 3.833 Å. Calculate the atomic radius of an iridium ...
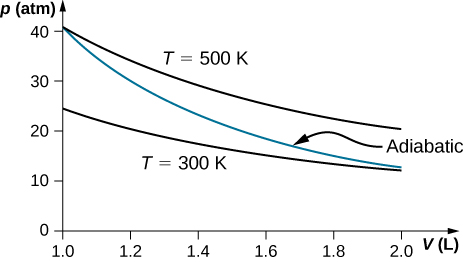
PDF Homework Chapter 22 Solutions - Squarespace Here is the diagram of the engine.!! (a)!Let's look at the values per minute. The process of the engine is adiabatic. At the initial state, !! The pressure and the temperature are known. The amount of argon is 80 kg. This is! ⎜ ! The initial volume then is!! At the final state, !!! 3 ! The relationship between the volume and temperature ... PDF First Law of Thermodynamics Heat and Work done by a Gas A P-V diagram question . An ideal gas initially in state 1 progresses to a final state by one of three different processes (a, b, or c). Each of the possible final states has the same temperature. For which process is the change in internal energy the largest? Answer: Because the final temperature is the same, the change in temperature is the Physics secondary stage 2 by Mohammed Talaat - Issuu The Gas Laws. Overview. It can be shown that gas molecules are in continuous random motion called Brownian. motion as follows: Heat. If we examine candle smoke through the microscope,we notice ... PDF Take Good Care of - aacaebc Figure 3.6 Sharing of electrons between hydrogen atoms in H 2 molecule. In the hydrogen molecule, each hydrogen atom attains the stable electron configuration of helium. In a covalent bond, each electron in a shared pair is attracted to the nuclei of both atoms as shown in Figure 3.6. The shared electrons spend most of their time between the ...
PDF Physics II Chapter 15 Practice Fall 2016 3.Calculate the total change in entropy of the universe that occurs when 0.2 kg of ice at 0 C melts in a 30 tub of ethanol. 4.A 0.03 mol sample of helium is taken through the cycle shown in the diagram above. The temperature of state A is 400 K. (a)For each process in this cycle, indicate in the table below whether the quantities W, Q, and U
Full text of "Fundamentals Of Classical Thermodynamics" An icon used to represent a menu that can be toggled by interacting with this icon.
Gases Assignment and Quiz Flashcards | Quizlet Pressure is the result of collisions of gas molecules with the walls of the tire. Temperature is a measure of the average kinetic energy of the gas molecules. As temperature increases, gas molecules move more quickly and they collide with the tire walls more frequently and with more force. Pressure and temperature are directly proportional.
(PDF) Polymerization. Edited by Ailton De ... - Academia.edu Academia.edu is a platform for academics to share research papers.
Full text of "A system of physical chemistry Due to a planned power outage on Friday, 1/14, between 8am-1pm PST, some services may be impacted.
Solved: Chapter 20 Problem 46P Solution - Chegg Step-by-step solution 100% (34 ratings) for this solution Step 1 of 4 Given Pressure and Volume and For monatomic ideal gas Heat capacity at constant pressure Heat capacity at constant volume Chapter 20, Problem 46P is solved. View this answer View a sample solution Step 2 of 4 Step 3 of 4 Step 4 of 4 Back to top Corresponding textbook
R134a Pv Diagram - apindustria.padova.it Locate the oil service valve and motor housing drain plug as per the following compressor diagrams in Figure 1. Fig 1: Vapour compression refrigeration cycle on p-h diagram The p-h diagram is frequently used in the analysis of vapour compression refrigeration cycle and usually consists of the four processes. ph diagram steam97 si megawatsoft.
Solved A gas undergoes a change of state described by the ... A gas undergoes a change of state described by the pVpV diagram shown in the figure below. Calculate the amount of work done on the gas. Answer is NOT: 4.00e2, 400, 40.0, 4000, or 200 Thanks! Show transcribed image text Expert Answer 100% (7 ratings)
INTRODUCTION TO MODERN STATISTICAL ... - Academia.edu Academia.edu is a platform for academics to share research papers.
(PDF) The characterization and dissolution ... - ResearchGate PVPVA ketoprofen 1. Introduction T oday, many ne wly identified active pharmaceutical ingredi- ents (API) are classified as low solubility, which give rise to a low bioavailability when administered...
Inhalational anaesthetics and n‐alcohols share a site of ... Several superimposed traces are shown before adding halothane, in the presence of 0.46 or 1 mM halothane and after washout (C, H and W respectively). (B) Bar graph summarizing the mean % change induced by two concentrations of halothane (0.46 or 1 mM). Negative and positive changes correspond to inhibition and potentiation respectively.
What are PV diagrams? (article) - Khan Academy Let's say our gas starts out in the state shown in the PV diagram below. If we press the piston downward, the volume of the gas will decrease, so the state must shift to the left toward smaller volumes (as seen in the diagram below). Since the gas is being compressed we can also say for sure that positive work is being done on the gas.
(PDF) The Influence of Drug Physical State on the ... Free energy-composition phase diagrams of OLZ and each polymer system (PVP, PVPVA, and SLP). +6 Differential scanning calorimetry curves of 50% drug-loaded formulations with SLP, PVPVA, and PVP...















0 Response to "38 a gas undergoes a change of state described by the pvpv diagram shown in the figure below."
Post a Comment