42 cf molecular orbital diagram
Asked for: molecular orbital energy-level diagram, valence electron... A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. We can therefore use a molecular orbital energy-level diagram and the calculated bond order to predict the relative stability of species such as H2+. Molecular Orbital Diagrams simplified | by Megan A. Lim | Medium Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories If you can understand the foundation and skeleton of the diagram specific to that molecule, then it will be easier and faster for you to draw it.
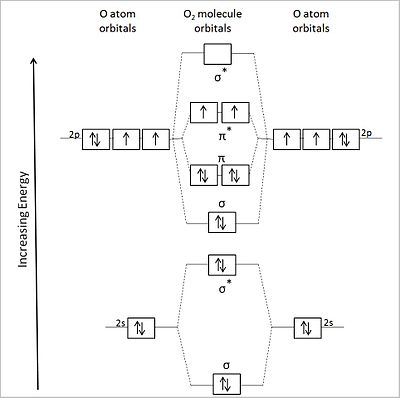
I.G Molecular Orbital Theory | ScienceDirect Topics Figure 3. Molecular orbital energy diagram for oxygen diatomic. Distinctive aspects of the representational strategies in the Lennard-Jones analysis are worthy of attention. A sophisticated version of this diagrammatic scheme remains in use even today as a qualitative predictive device.
Cf molecular orbital diagram
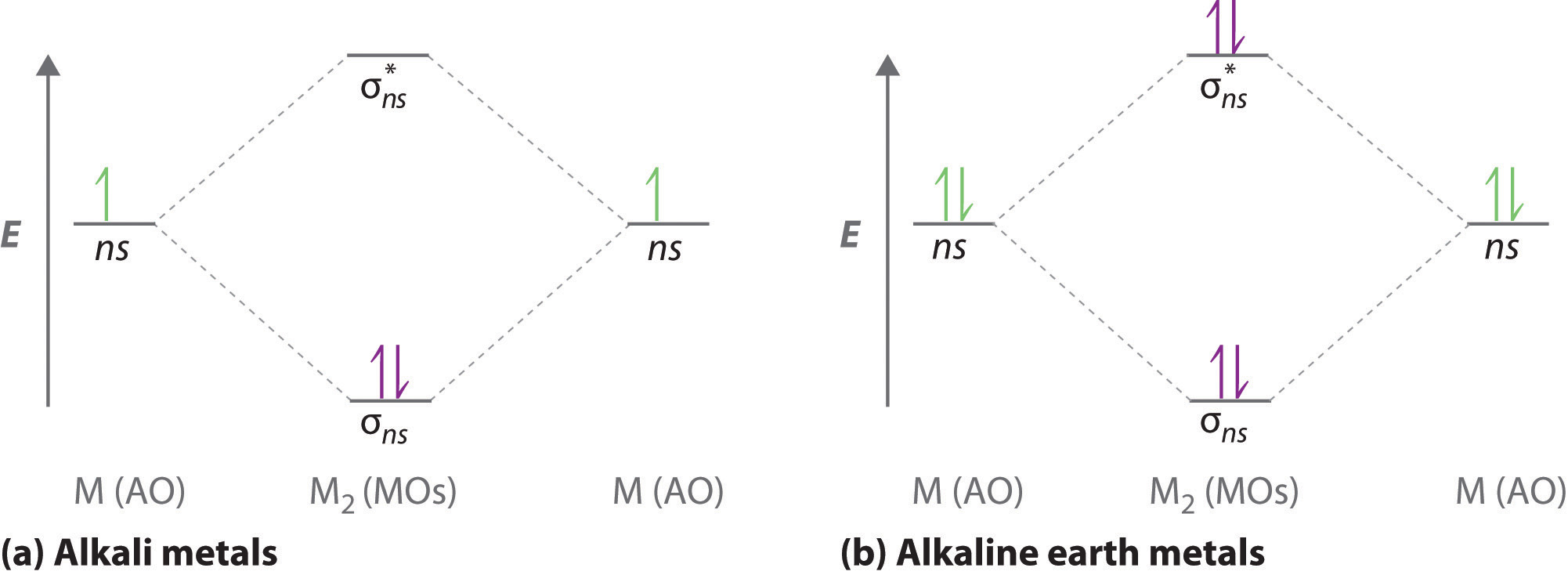
PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... • Molecule orbital theory (Robert Mullikan). • Electrons are delocalised - Different to Lewis and hybridisation (these are not MO). • Energy level diagram represents this interaction. - Two s orbitals interaction to create a low energy bonding and high energy anti-bonding molecular orbital. What is the molecular orbital diagram of O2 and F2? - Quora The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used them up. They completely fill all the orbitals except the highest-energy antibonding sigma 2p orbital. 8.4 Molecular Orbital Theory - Chemistry The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. Figure 10. The molecular orbital energy diagram predicts that He2 will not be a stable molecule, since it has equal numbers of bonding and antibonding electrons.
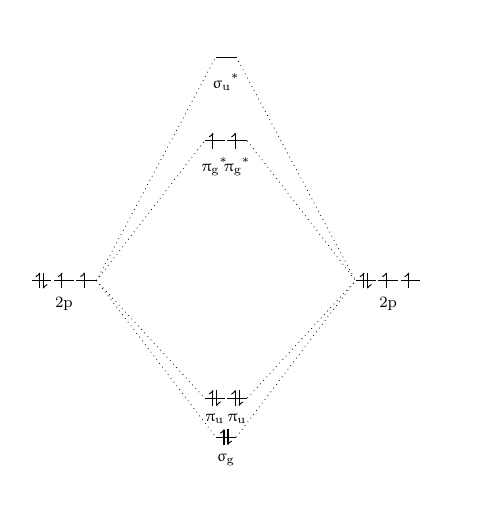
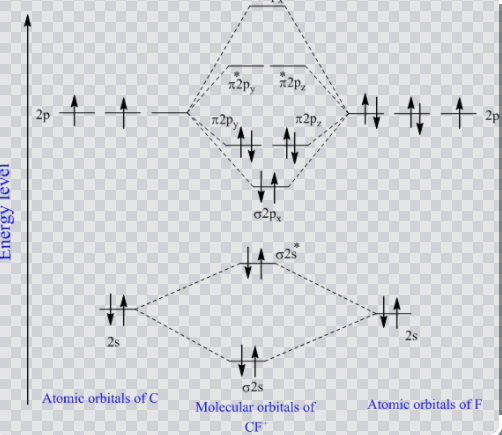
Cf molecular orbital diagram. Molecular orbital diagrams - Overleaf, Online LaTeX Editor Molecular orbital diagrams provide qualitative information about the structure and stability of the electrons in a molecule. This article explains how to create molecular orbital diagrams in LaTeX by means of the package MOdiagram. For information about the more traditional molecular structure... Introduction to Inorganic Chemistry/Molecular Orbital Theory... Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons. SOLVED:Construct the molecular orbital diagram fo Problem 39 Medium Difficulty. Construct the molecular orbital diagram for CF. Would you expect the bond length of $\mathrm{CF}^{+}$ to be longer or shorter than that of CF? To both CF and CF' ions, CF' has shorter bond length than CF. This is because CF' bond order more than CF molecule. Chapter 9 Molecular Orbitals in Chemical Bonding (Midterm) | Quizlet draw the molecular orbital diagram for B2. the number of electrons in the pi2p molecular orbital is. their molecular orbital diagrams are more symmetrical than those of homonuclear diatomic molecules. which of the following statements about nitrogen oxide, NO, is FALSE.
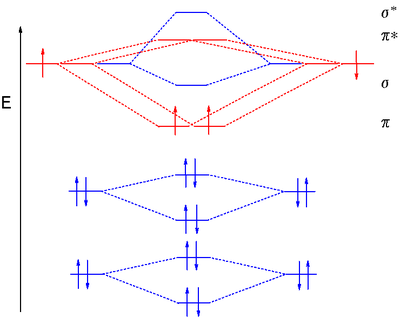
8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Creating molecular orbital diagrams for molecules with more than two atoms relies on the same basic ideas as the diatomic examples presented here. However, with more atoms, computers are required to calculate how the atomic orbitals combine. See three-dimensional drawings of the... Molecular Orbital diagram of NO(nitric oxide) molecule Molecular orbital : A molecule in which all the electrons are paired, is called diamagnetic. | Online Chemistry tutorial IIT, CBSE Chemistry, ICSE Chemistry, engineering and medical chemistry entrance exams Molecular orbital diagram of C2 molecule : Number of electrons in C2 molecule = 12. PDF Microsoft Word - Chapter 1_6_SY.doc Molecular Orbital Diagrams (H2 and He2) One of the strengths of molecular orbital theory is its ability to describe the energy of both occupied and unoccupied molecular orbitals for a molecule. A molecular orbital diagram shows both the energy of the atomic orbitals (from the atoms that are... Molecular Orbital Theory | Boundless Chemistry Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the...
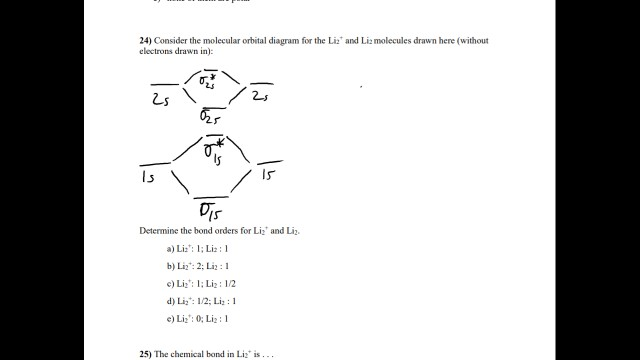
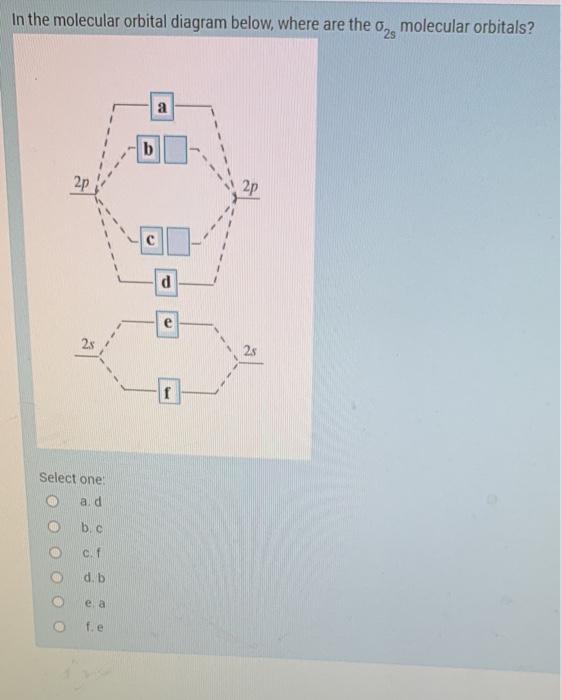
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. Hybridization 4 - Lecture notes 1 - MOLECULAR ORBITAL DIAGRAM... Molecular orbital diagram key. Draw molecular orbital diagrams for each of the following molecules or ions. Determine the bond order of each and use this to predict the stability of the bond. Molecular Orbital Theory: Explanation, Illustrations and... - Embibe Molecular Orbital Theory: To simplify things, we will consider the interaction of the orbitals containing valence electrons to create molecular orbitals. Have you ever thought about how sigma and pi bonds are formed? What is the difference between diamagnetic and paramagnetic behaviour? PDF Chapter 5 | 5.2.2 Orbital Mixing Molecular orbital theory uses group theory to describe the bonding in molecules; it comple-ments and extends the introductory bonding models in Chapter 3 . In molecular orbital theory the symmetry properties and relative energies of atomic orbitals determine how these orbitals interact to form...
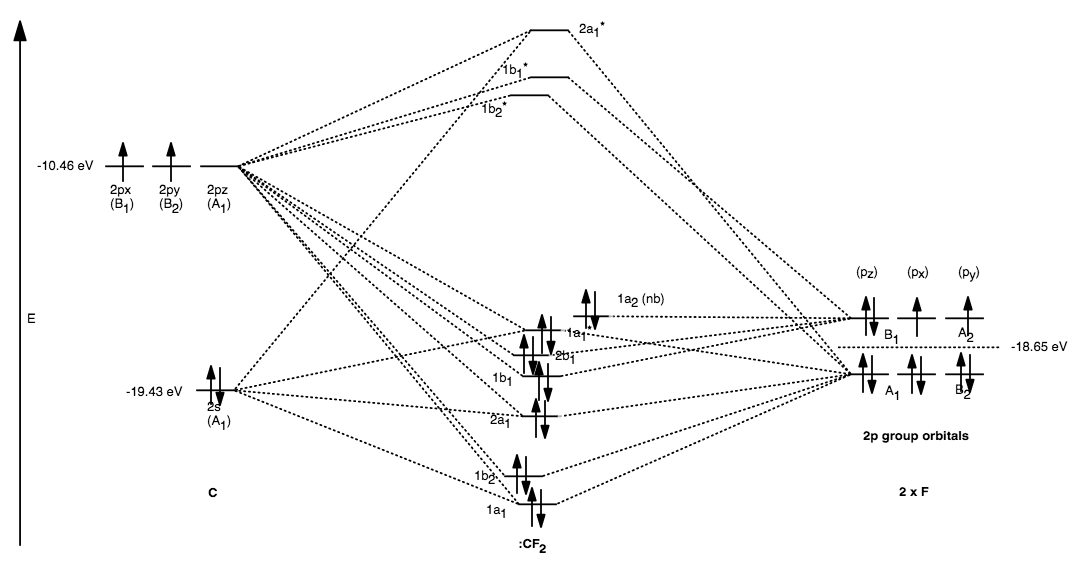
molecular orbital diagram key 365 MOLECULAR ORBITAL DIAGRAM KEY Draw molecular orbital diagrams for each of the following molecules or ions. a. CF4 ' $ '. hybridization for the C sp3 hybridization for each F sp3 There are four sigma bonds due to sp3-sp3 overlap. electron group geometry around the C...
An introduction to molecular orbital theory The molecular orbital model is by far the most productive of the various models of chemical bonding, and serves as the basis for most quantiative Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its positive...
according to the increasing order of CF bond length: (A) \[C{F ... Total electron present in the CF molecule = 6 + 9 = 15 electrons. In the molecular orbital diagram of CF molecule, the electrons are arranged in the following ...1 answer · Top answer: Hint: In order to find the increasing order of the bond length of the species \[CF\], \[C{F^ + }\] and \[C{F^ - }\] of the C-F molecule, we must be ...
Chem 344 - Homework 9 – due Friday, Apr. 11, 2014, 2 PM 11 Apr 2014 — P16.5) The bond dissociation energies of the species NO, CF ... Make a molecular orbital diagram for this molecule, associate the MOs with ...13 pages
PDF Molecular | 90" (porbitals). This dilemma has been resolved by orbital Notes on. Molecular Orbital ~ a l c u l aitons. Notes on molecular orbital calculations. First printing, 1961 Second printing, with corrections, 1962 Third 'Cf. C. A. Coulson, Q u a r t e r l y Reviews, 144 (1947). 2 ~ P.auling, "Nature of the Chemical Bond, " pp. 14-15...
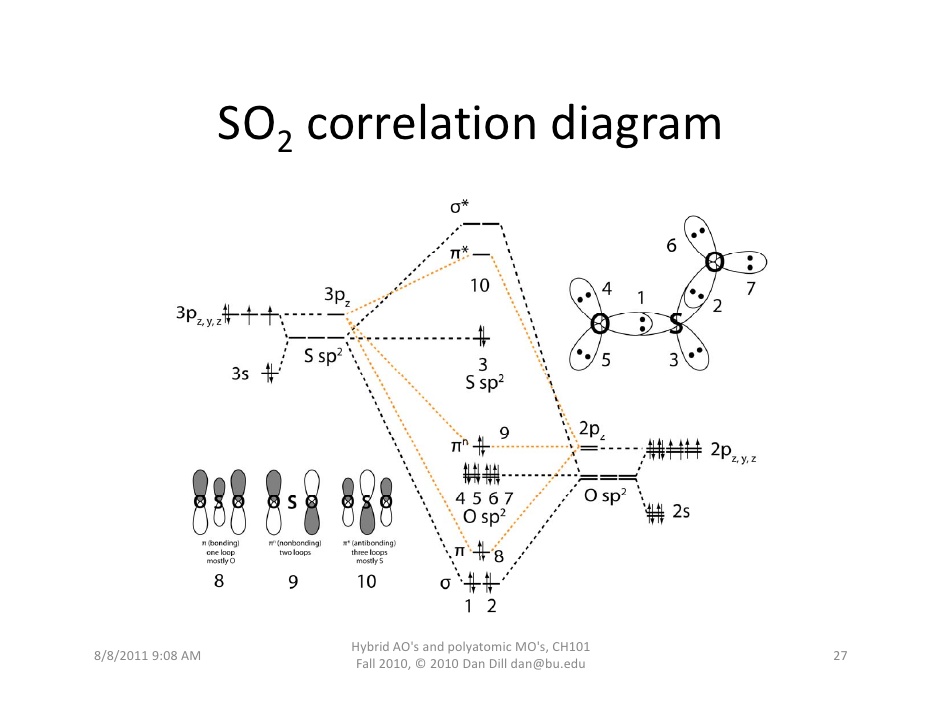
PDF Microsoft Word - Handin8s2017ans.docx 1. Sketch the qualitative molecular orbital diagram for XeF2. The molecule is linear and symmetric. Assume the valence 5s-orbitals of Xe are sufficiently lower in energy than the valence Does your MO diagram agree with this expectation? Determine the primary MOs that determine the bond order.
Molecular Orbital Diagrams -- Chemistry X - YouTube For chem videos, quizzes and more download Chemistry X for free on the App Store! Correlation Diagrams - by considering the positions and energies of...
Molecular Orbital Theory: Energy level diagram for molecular orbitals Molecular orbital theory was put forward by Hund and Mullikan in 1932. This theory is modern and more rational. This theory assume that in molecules, atomic orbitals lose their identity and the electrons in molecules are present in new orbitals called molecular orbitals.
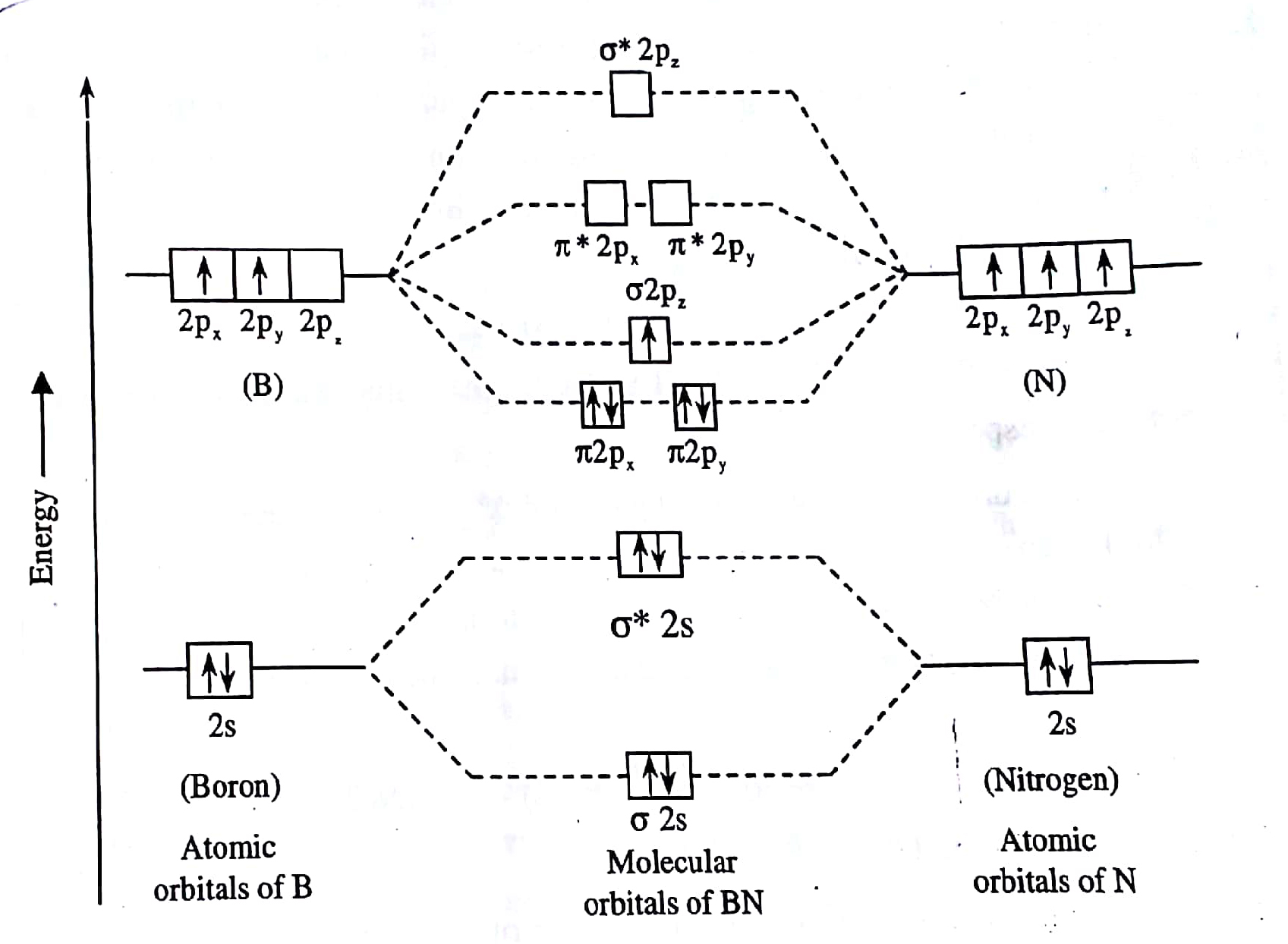
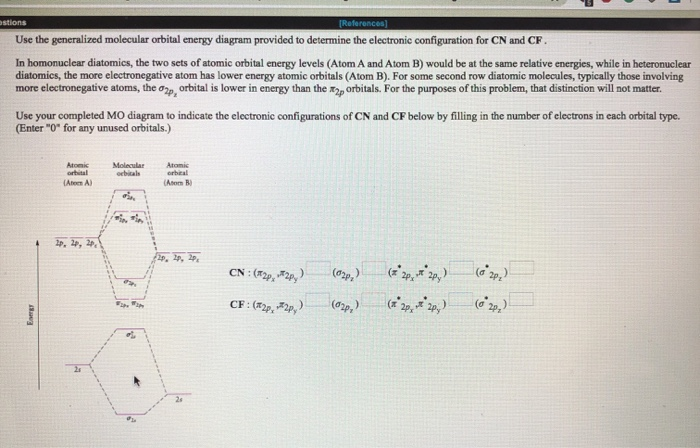
PDF Molecular Orbitals in | 9-2 Molecular Orbital Energy Level Diagrams Accordingly, a molecular orbital diagram such as Figure 9-5 is inappropriate for heteronuclear diatomic molecules. If the two elements are similar (as in NO or CN mole-cules, for example), we can modify the diagram of Figure 9-5 by skewing it slightly.
MO Diagrams | Molecular Orbital Diagram Maker A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row
Molecular Orbital Theory (MOT), Chemistry Study... | eMedicalPrep The molecular orbital diagram representing this order of energy levels is shown in fig. No. 9 Molecular Orbital Diagram for CO. Analysis done by Bond Order. If value of bond order is positive, it indicates a stable molecule and if the value is negative or zero, it means that the molecule is unstable.
FIG. 1. Molecular orbital diagrams for the valence shells of (a) CF 4... ... corresponding molecular orbital diagrams, depicting the dominant atomic orbital characters of the occupied and some of the low-lying unoccupied (virtual) The major contribution to CF3⁺ formation originates from ionization of the 4t2 orbital while CF2⁺ is mainly formed after 3t2 orbital ionization.
Molecular Orbital Theory - Chemistry | Socratic Molecular orbital theory is a method for determining molecular structure. It describes electrons as moving under the influence of the nucleus and not Molecular Orbital (MO) theory better explains the properties of more complex molecules. MO theory explains the partial bonds of NO₃⁻ without using...
Molecular Orbital Theory Molecular orbital theory is more powerful than valence-bond theory because the orbitals reflect the The bonding molecular orbital concentrates electrons in the region directly between the two nuclei. The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both...
8.4 Molecular Orbital Theory - Chemistry The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. Figure 10. The molecular orbital energy diagram predicts that He2 will not be a stable molecule, since it has equal numbers of bonding and antibonding electrons.
What is the molecular orbital diagram of O2 and F2? - Quora The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used them up. They completely fill all the orbitals except the highest-energy antibonding sigma 2p orbital.
PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... • Molecule orbital theory (Robert Mullikan). • Electrons are delocalised - Different to Lewis and hybridisation (these are not MO). • Energy level diagram represents this interaction. - Two s orbitals interaction to create a low energy bonding and high energy anti-bonding molecular orbital.




















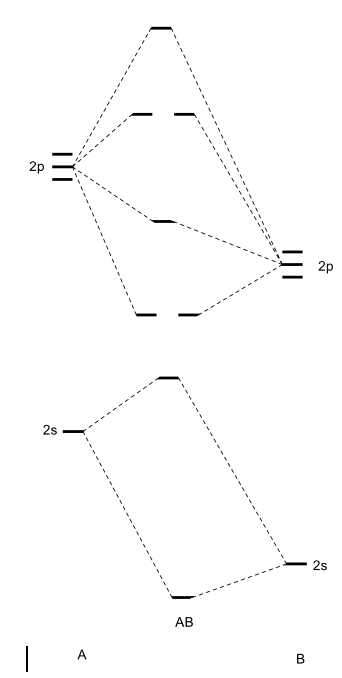

0 Response to "42 cf molecular orbital diagram"
Post a Comment