40 orbital diagram of oxygen
Atomic and Molecular Orbital Diagram for Oxygen/O2 Periodic Table of Elements. Discuss: orbital energies, orbital filling, magnetism. molecular electron configurations. Compare: Sum of # of atomic orbitals Molecular anti-bonding orbitals higher in energy than atomic orbitals, contributes to molecular instability. Molecular Diagram of B C N (Vs) O F Ne... Molecular Orbital Theory This diagram suggests that the energy of an H2 molecule is lower than that of a pair of isolated atoms. The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals.
PDF Figure 2: Molecular oxygen energetic diagram of O2. ...diagram of the oxygen atom because similarities with the energy diagram of molecular oxygen can also be used to explain the reactivity of molecular oxygen. can be constructed from the molecular orbital theory (Figure 2). In molecular oxygen, there are 16. electrons which can be placed into the...

Orbital diagram of oxygen
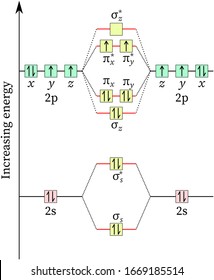
Molecular Orbital Energy Diagrams Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O2. From the molecular orbital diagram of N2, predict its bond order and whether it is diamagnetic or paramagnetic. Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. 8.4: Molecular Orbital Theory - Chemistry LibreTexts Draw the molecular orbital diagram for the oxygen molecule, O 2 . From this diagram, calculate the bond order for O 2 . How does this diagram account for the paramagnetism of O 2 ?
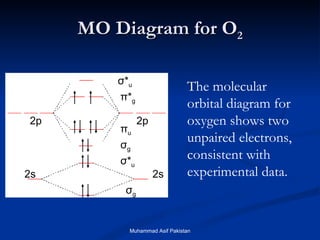
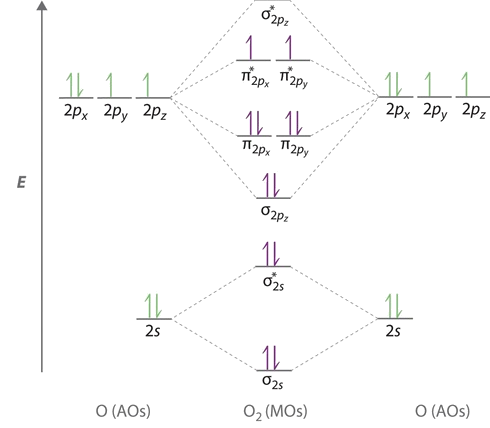
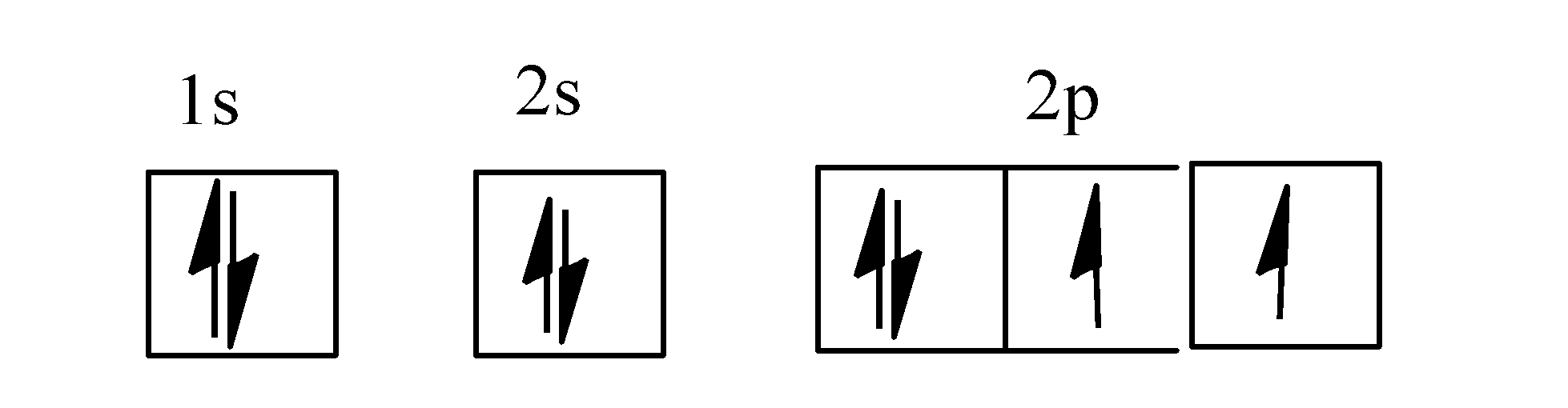
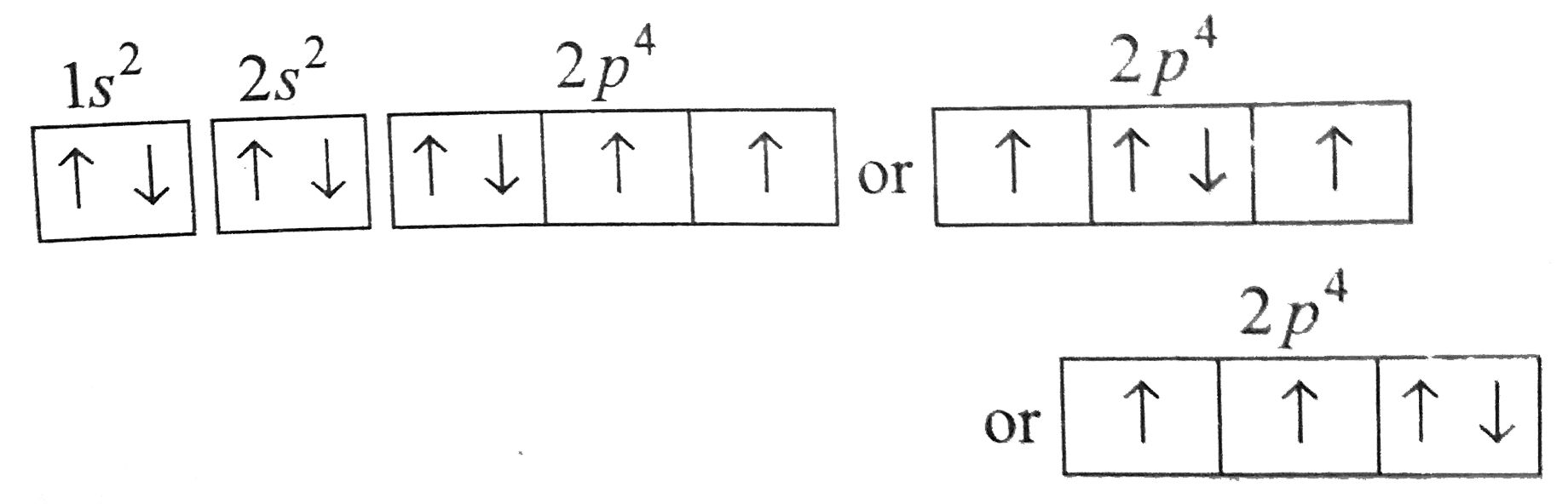
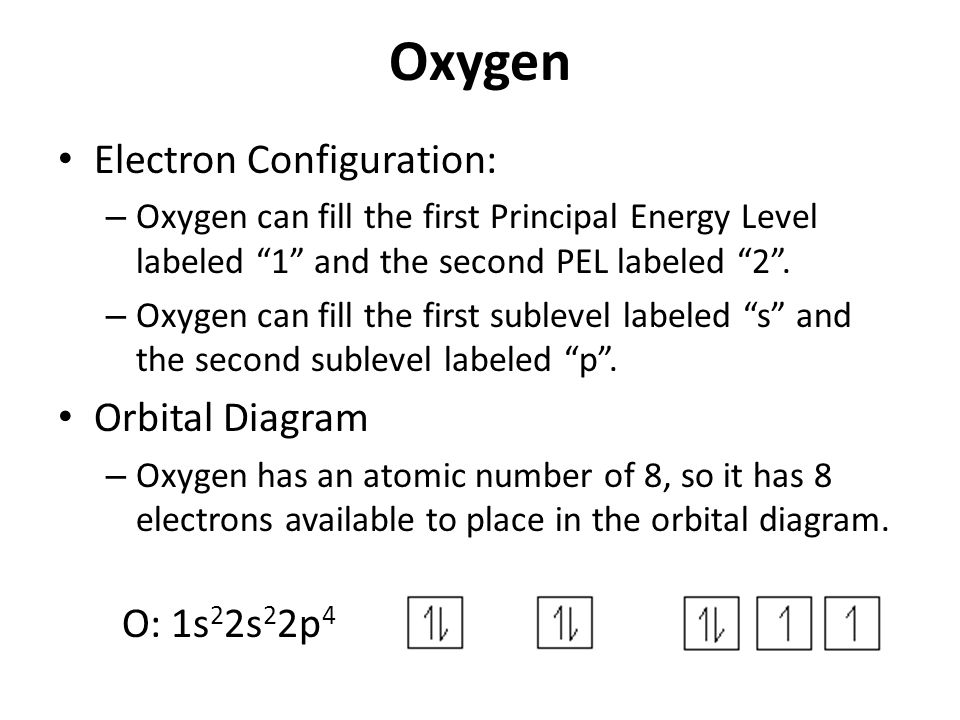
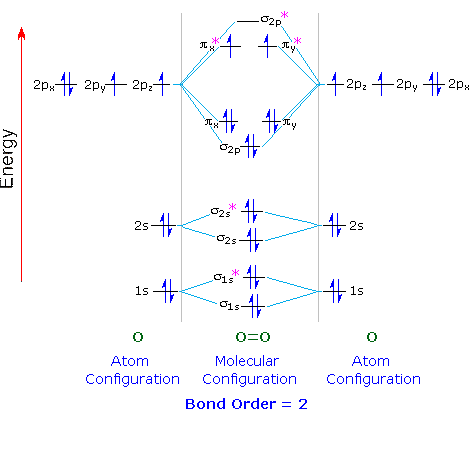
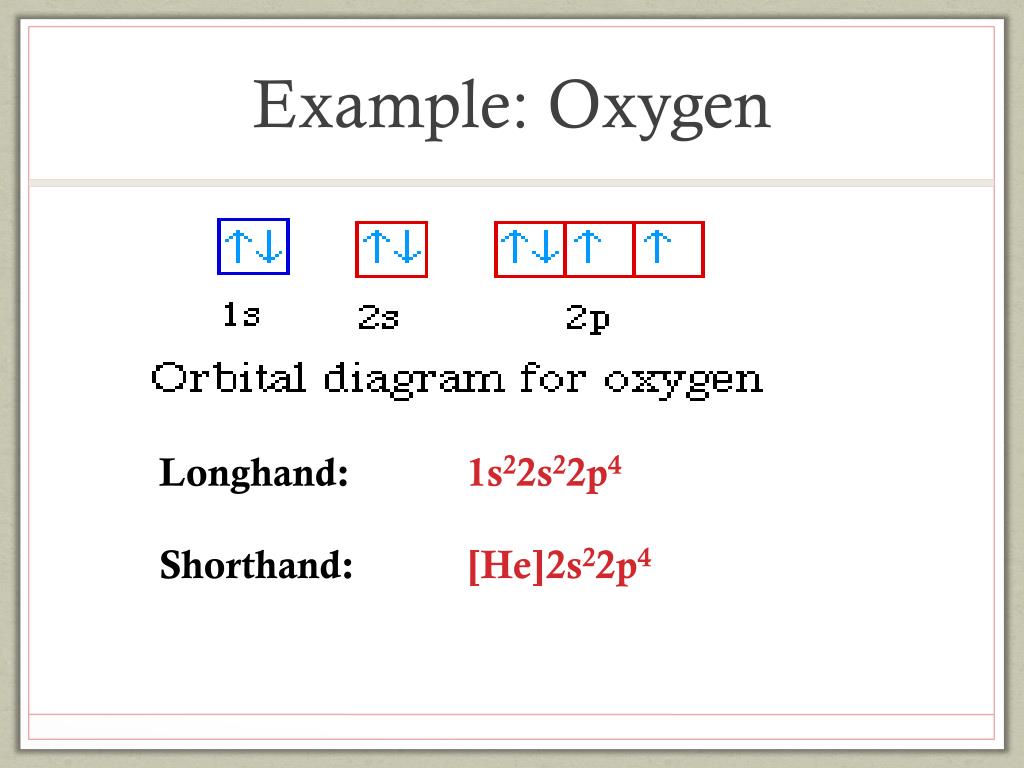
Orbital diagram of oxygen. Oxygen(O) electron configuration and orbital diagram Oxygen(O) is the 8th element in the periodic table and its symbol is 'O'. This article gives an idea about the electron configuration of oxygen and orbital diagram, period and groups, valency and valence electrons of oxygen, bond formation, compound formation, application of different principles. Orbital Diagrams — Overview & Examples - Expii An orbital diagram, or orbital filling diagram, is a type of notation which illustrates an atom's electron distribution and electron spin within orbitals. The Basics of Orbital Diagrams. There are different types of orbitals, that all have different energy levels. These orbitals are filled with electrons (the... Orbital Diagrams Chemistry Tutorial Orbital Diagrams Chemistry Tutorial. Key Concepts. An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration (using the Aufau Principle to order the orbitals and hence... 8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Draw the molecular orbital diagram for the oxygen molecule, O2. From this diagram, calculate the bond order for O2. Each oxygen atom contributes six electrons, so the diagram appears as shown in Figure 8.40. Figure 8.40 The molecular orbital energy diagram for O2 predicts two unpaired electrons.
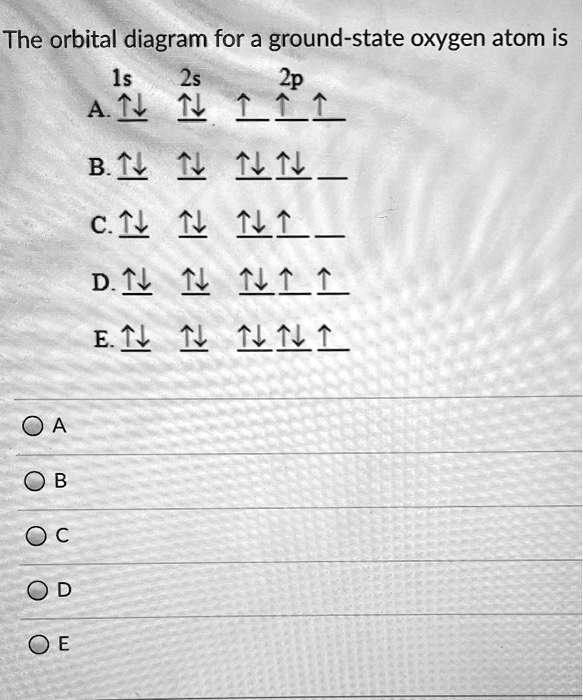
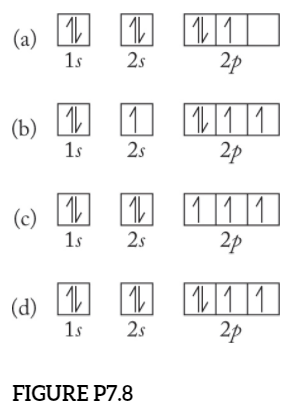
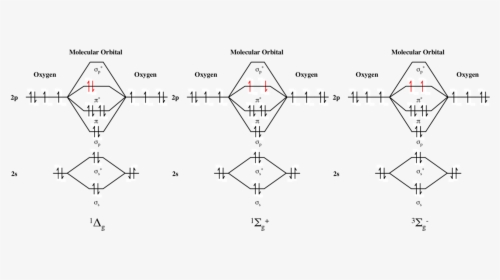
High School Chemistry/Orbital Configurations - Wikibooks, open books... The orbital diagram on the left is the correct orbital diagram, because it obeys Hund's Rule, meaning that there is less electron-electron repulsion and, as a result, the electrons have lower energies (remember Draw the orbital diagram for oxygen (O). Use it to answer the following questions Molecular orbital energy level diagrams -Hydrogen, Hypothetical... The molecular orbital energy level diagram of N2 is given in Fig.. The bond order of N2 can be calculated as follows. Here, Nb = 8 and Na = 2. The electronic configuration of oxygen (Z = 8) in the ground state is 1s22s22p4. Each oxygen atom has 8 electrons, hence, in O2 molecule there are 16... Electron Configuration | Boundless Chemistry Orbital diagram: The positions of the first ten orbits of an atom on an energy diagram. Note that each block is able to hold two electrons. Application of Hund's rule: Orbital diagram for oxygen, which has four 2p electrons, showing the correct application of Hund's Rule. 13 Molecular orbital diagram of oxygen molecule. Reproduced from... Download scientific diagram | 13 Molecular orbital diagram of oxygen molecule. The oxygen reduction reaction (ORR) process in the cathode compartment occurs by the direct transfer pathway of 4-electrons from O2 to H2O (O2 + 2H2O + 4e − → 4 OH − , E0′ = 0.815 V, pH = 7) [30] .
Oxygen Definition, Facts, Symbol, Discovery, Property, Uses Oxygen (pronunciation: OK-si-jen) is a colorless element that belongs to the group of Chalcogens in the periodic table, and it is represented by the chemical symbol O [1, 2, 3]. A highly reactive non-metal, it can easily form oxides with most of Oxygen Molecular Orbital Diagram. Biological Role of Oxygen. 8.4 Molecular Orbital Theory - Chemistry Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O2. From the molecular orbital diagram of N2, predict its bond order and whether it is diamagnetic or paramagnetic. Solved Complete the valence molecular-orbital diagram for | Chegg.com Transcribed image text : Complete the valence molecular-orbital diagram for oxygen, 0, 1,00 JT Žp 2p Answer Bank 02pm 21 Os. Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Atomic no.
What is the molecular orbital diagram for oxygen? - Quora From the molecular orbital diagram, we observe that oxygen has two unpaired electrons which is consist with the paramagnetic nature of oxygen. The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in...
Molecular Orbital Diagram of Oxygen Molecule - Nature of Chemical... Molecular Orbital Diagram of Oxygen Molecule Video Lecture from Chapter Nature of Chemical Bond of Subject Chemistry Class 11 for HSC, IIT JEE, CBSE...
Oxygen orbital diagram - Big Chemical Encyclopedia Figure 1.7 Molecular orbital diagram for molecular oxygen, O2. From K. M. Ralls, T. H. Courtney, and J. Wulff, Introduction to Materials Science and Engineering. Figure 6.5 Molecular orbital diagram of MOg cluster formed by a transition metal with oxygen.
Oxygen Electron Configuration (O) with Orbital Diagram Oxygen Electron Configuration. The symbol for Oxygen is O. It is one of the members of the chalcogen group in the periodic table and also a highly reactive There is Six valence electron in the Oxygen. You can see the above Orbital Dot Diagram. The symbol of Oxygen is O and the atomic Mass of...
What is the orbital diagram for oxygen? - Answers An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down A molecular orbital diagram is used to map out locations of molecules. Just like planets orbiting around the sun, molecules orbit around particles.
File:Oxygen molecule orbitals diagram.JPG - Wikimedia Commons It is recommended to name the SVG file "Oxygen molecule orbitals diagram.svg" - then the template Vector version available (or Vva) does not need the new image name parameter. DescriptionOxygen molecule orbitals diagram.JPG. English: Molecular orbital energy diagram for O2.
Explain the formation of O2 molecule using molecular orbital theory. The molecular orbital energy level diagram of oxygen molecule is given as follows : Bond order 2Nb −Na =28−4 =2 Thus, oxygen molecule has two bonds. i.e., one is bond and one p bond. The last two electrons in p2px∙ and p2py∙ orbitals will remain unpaired. Therefore, oxygen molecule has...

Draw the valence shell molecular orbital diagram of oxygen molecule and predict its magnetic nature.
How do yo write the orbital diagram for oxygen? | Socratic The electron configuration for oxygen is: 1s^2 2s^2 2p^4 This video will walk you through the step of writing orbital diagram. The video uses Kr as an example, but the process is exactly as the same as what you need to do for oxygen. Hope this helps!
PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... - Oxygen - hydrogen interactions share 2 electron Æ H ─ O. - Oxygen also has two lone pairs. LUMO - 2π* Comes from standard π interaction however lower oxygen orbital means π has has more details of the bonding diagram between the Lewis and MO treatments. 4. Perform the same...
Electron configuration for Oxygen (element 8). Orbital diagram O (Oxygen) is an element with position number 8 in the periodic table. Located in the II period. Melting point: -218.4 ℃. Density: 0.00133 g/cm3. Below is the electronic diagram of the Oxygen atom. Distribution of electrons over energy levels in the O atom 1-st level (K): 2 2-st level (L): 6.
Atomic Orbital Diagram for Oxygen | Online Chemistry Help The above diagram explains the molecular orbital energy level diagram for molecules of Oxygen and other heavier elements. However, experimental evidence for oxygen and heavier diatomic molecules has shown that above sequence of energy levels of MOs is not correct. In case of these elements, the...
Orbital Diagrams & Electron Configurations for Atoms and Ions Draw an orbital diagram for beryllium (Z=4) 1s Guidelines for drawing orbital diagrams 1s 1. Fill orbitals in order of increasing energy. 1s 2s 7 N nitrogen 14.01 2p Ti 8 O oxygen 16.00 Orbital diagrams for ions • anion (negative charge): ADD appropriate number of electrons • cation (positive)...
8.4: Molecular Orbital Theory - Chemistry LibreTexts Draw the molecular orbital diagram for the oxygen molecule, O 2 . From this diagram, calculate the bond order for O 2 . How does this diagram account for the paramagnetism of O 2 ?
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
Molecular Orbital Energy Diagrams Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O2. From the molecular orbital diagram of N2, predict its bond order and whether it is diamagnetic or paramagnetic.




![Expert Verified] Show the distribution of electrons in oxygen ...](https://hi-static.z-dn.net/files/d13/1581d81d71e4d32ff6e46ba056805907.png)















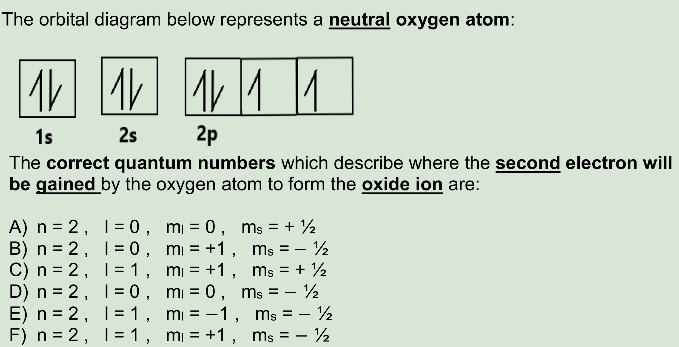

0 Response to "40 orbital diagram of oxygen"
Post a Comment