39 phase change diagram for rubbing alcohol
Phase Equilibrium | slideum.com • If you open rubbing alcohol, you can smell it. This is because the alcohol diffuses out in the _____ form. • After you recap the alcohol, an equilibrium is quickly reached again. • A dynamic EQUILIBRIUM is reached when particles are entering the gaseous phase at an equal rate as the particles go back to the liquid phase. Isopropyl alcohol | (CH3)2CHOH - PubChem Isopropyl Alcohol is an isomer of propyl alcohol with antibacterial properties. Although the exact mechanism of isopropanol's disinfecting action is not known, it might kill cells by denaturing cell proteins and DNA, interfering with cellular metabolism, and dissolving cell lipo-protein membranes.
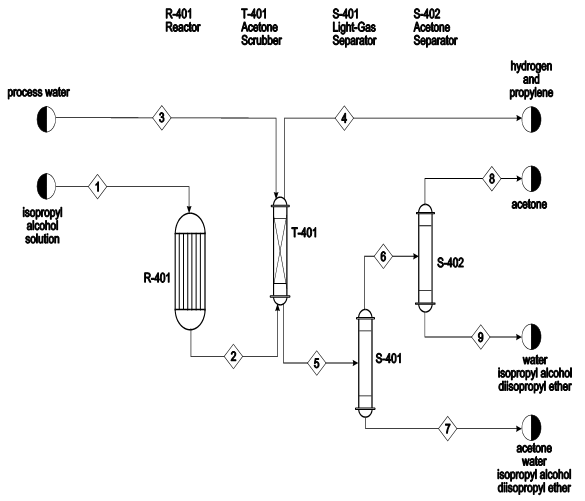
PDF Refrigeration and Air Conditioning Using Alcohol As a ... Inthis cycle certain changes with the componets are made which are as follows: 2.1. Phase Changer As the name suggests, a Phase changer (Fig.2) is device in which the phase of alcohol is changed from liquid to gaseous form. In this system, a closed tank is used to make a chamber for hot water, there is a copper pipe coming from the pump,
Phase change diagram for rubbing alcohol
phase change study guide.doc - Name:_ Date:_ Period ... The change from liquid water to steam (a gas) is a change in phase and requires the gain of heat energy. This energy can be gained (taken in) from the environment. When you put rubbing alcohol on your skin, it makes your skin feel cold. PDF the Energy of Evaporation | A Lab Investigation evaporation is a phase change that absorbs energy. During evaporation, some fast-moving, highly energetic molecules have enough energy to over-come the attractions that individual molecules have for one another and enter the gas phase. As these high-energy molecules leave the liquid phase, the average energy of the remaining liquid Isopropyl Alcohol - NIST Isopropyl Alcohol. Formula: C 3 H 8 O. Molecular weight: 60.0950. IUPAC Standard InChI: InChI=1S/C3H8O/c1-3 (2)4/h3-4H,1-2H3. Copy Sheet of paper on top of another sheet. IUPAC Standard InChIKey: KFZMGEQAYNKOFK-UHFFFAOYSA-N. Copy Sheet of paper on top of another sheet.
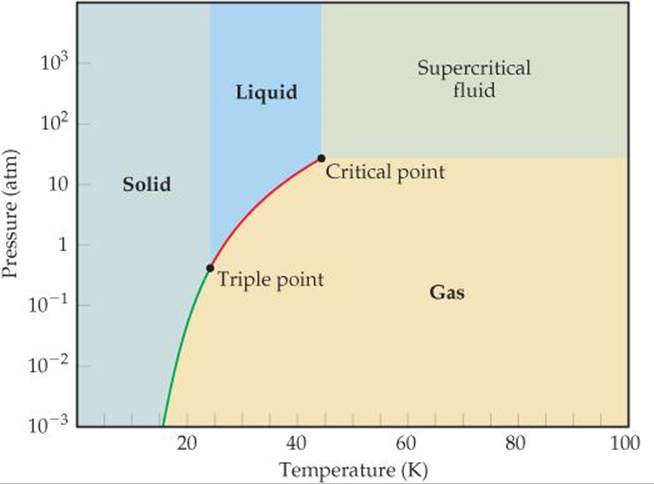
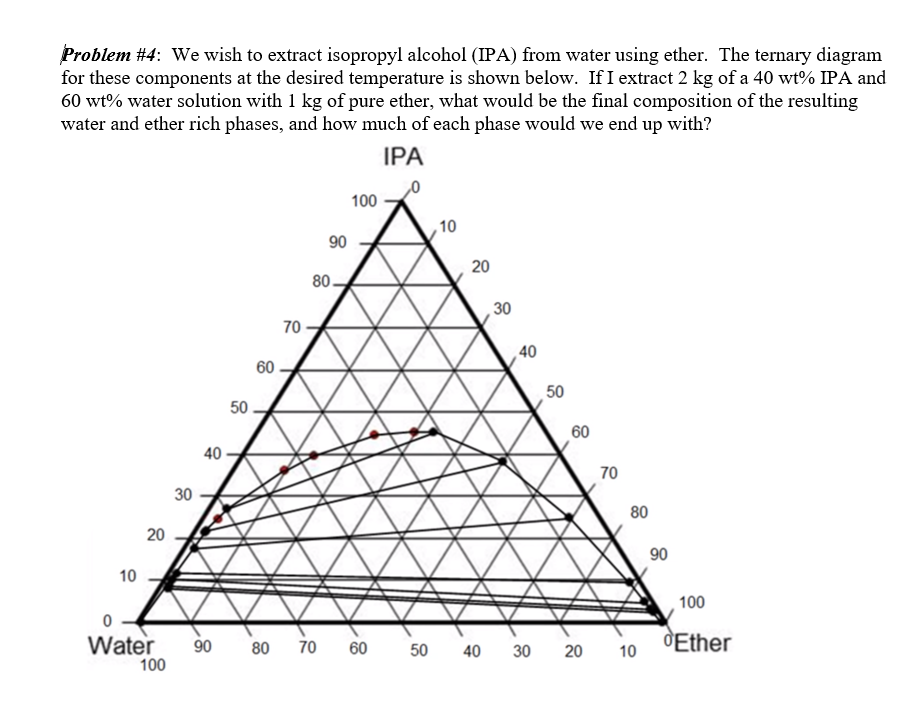
Phase change diagram for rubbing alcohol. Rubbing alcohol (isopropanol) is usually sold as a 70%vol ... Rubbing alcohol (isopropanol) is usually sold as a 70%vol aqueous solution. If the density of isopropyl alcohol is 0.785 g/mL, how many grams of isopropyl alcohol are present in a 355 mL bottle of rubbing alcohol? ... Phase Change Diagram_Water Question 16 options: The kinetic energy is increasing bec … ause the molecules are speeding up. The ... What is tie line in chemistry? - michaelemilio 2 shows the typical features of a ternary phase diagram for a system that forms a liquid and a vapor at fixed temperature and pressure. Mixtures with overall compositions that lie inside the binodal curve will split into liquid and vapor. Tie lines connect compositions of liquid and vapor phases in equilibrium. Phase Changes.pptx - Source: rustusovka.com Source: www ... Rubbing alcohol has a lower heat of vaporization than water, so the heat from your hands is enough to increase the KE of the alcohols and evaporate them. As a result of the loss of heat, your hands feel cold. Source: ... PHASE CHANGES • PHASE DIAGRAM Enables us to: ... PDF Unit 1: Evaporation- "Evaporative Cooling" level representation of the water and rubbing alcohol molecules at -6 degrees Celsius. Item 6. Using the drawings above, explain when Kellie took out the tray from the freezer, the water was still mostly solid but the rubbing alcohol was liquid. Focus your explanation on the relative strength of the electric forces between molecules.
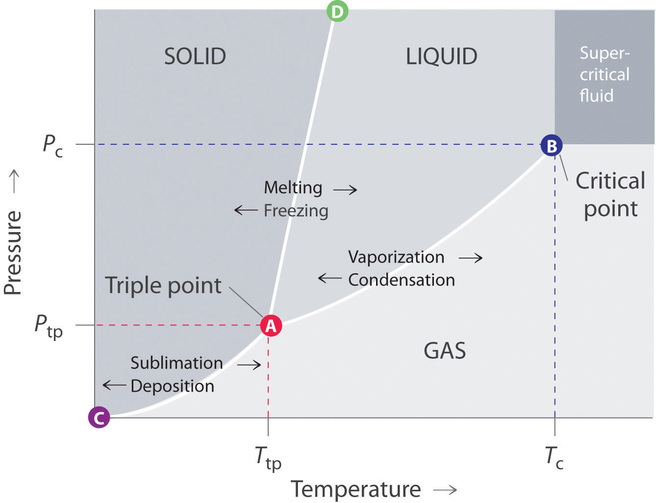
PDF Scanned Document Which phase change results in the release of energy? A) H20(g) B) H20(g) O, 1-120(1) C) H20(g) ... diagram the products in the given box [Indicate the exact arrangement of the particles you diagram.] ... Two alcohols that are used in our everyday lives are rubbing alcohol and ethylene glycol. Rubbing alcohol is used as an antiseptic. DOC STUDY GUIDE - Montgomery Township School District The change from liquid water to steam (a gas) is a change in phase and requires the gain of heat energy. This energy can be gained (taken in) from the environment. When you put rubbing alcohol on your skin, it makes your skin feel cold. The Energy of Evaporation | Energy Foundations for High ... In this activity, you will explore the energy change that accompanies the process of evaporation. Evaporation, like melting or freezing, is an example of a phase change—a change from one physical form of a substance to another.. During evaporation, energetic molecules leave the liquid phase, which lowers the average energy of the remaining liquid molecules. PDF Phase Diagrams States of Matter and Phase Changes Phase diagrams are used to show when a specific substance will change its state of matter (alignment of particles and distance between particles). Every substance has its own phase diagram. Some are very complex while others are simple.
Answered: phase change: | bartleby Question. Transcribed Image Text: Match the following change of enthaplies to their correct phase change: freezing 1. fusion (+ value) melting 2. vaporization (+ value) boiling 3. fusion (- value) 4. vaporization (- value) condensation. Expert Solution. FTIR spectra of ITO glass cells with rubbed PVA alignment ... A polyvinyl alcohol (PVA) alignment layer, which gives a very low pretilt angle when in contact with the liquid crystal (LC), was reacted with trifluoroacetic anhydride (TFAA) in the gas phase to ... Chem - ch12 (phase changes) Flashcards | Quizlet the process by which a gas changes directly to a solid without first becoming a liquid. phase diagram. a graph of pressure versus temperature that shows which phase a substance exists in under different conditions of temperature and pressure. hydrogen. heat energy disrupts the _ used to raise the temperature. temperature. Does acetone leave residue after evaporation ... Rubbing alcohol, also called isopranol, is less volatile meaning that it doesn't evaporate as easily. ... However, at low temperature and/or very high pressures it becomes a solid. The phase diagram for acetone shows the phase behavior with changes in temperature and pressure. ... The phase diagram for acetone shows the phase behavior with ...
Chemistry: Phase Changes - EntryTest Looking at the phase change diagram for water and following the dashed line at 1 atm, you can see that water would begin as a solid (ice) and melt at 0ºC. All of the water would be in liquid form by the time the temperature reached 75ºC. The second type of phase change graph you might see on the SAT II Chemistry exam is called a heating curve.
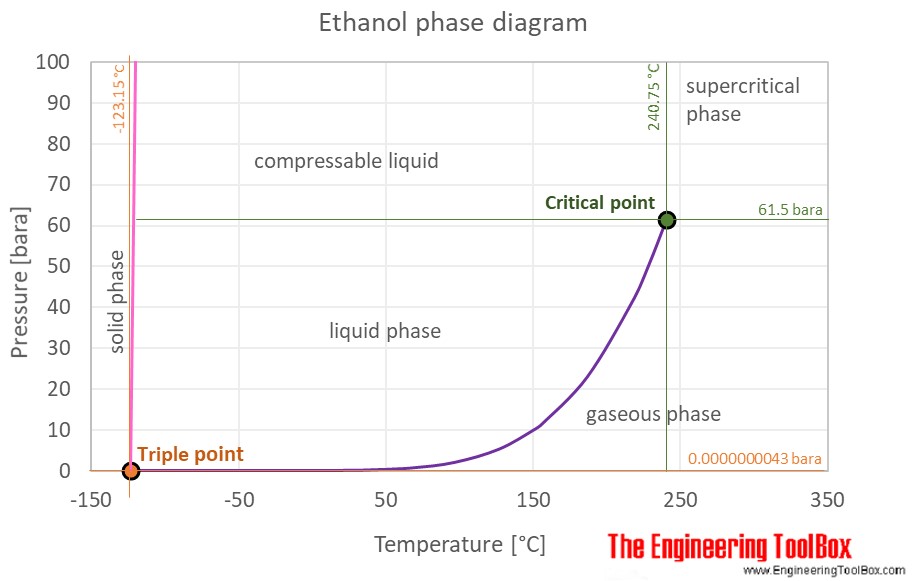
Ethanol - Thermophysical properties - Engineering ToolBox Chemical, physical and thermal properties of ethanol (also called alcohol or ethyl alcohol). Phase diagram included. Ethanol (Ethyl Alcohol), C2H5OH, is a volatile, flammable, colorless liquid with a slight characteristic odor. It is produced via petrochemical processes or naturally by the fermentation of sugars by yeasts.
Answered: Intermolecular Forces: Phase Changes &… | bartleby Science Chemistry Q&A Library Intermolecular Forces: Phase Changes & Properties Objectives: 1. Interpret the heating curve of substance X 2. Construct the cooling curve of a pure substance using experimental data. 3. Use phase diagram of pure substance to determine its phase at a given temperature and pressure 4.
Matter Flashcards | Quizlet Start studying Matter. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
PDF Freezing Point Depression - University of Kansas At a given pressure, the temperature at which a specific pure substance undergoes a particular phase change (freezing, boiling, sublimation, etc.) is always the same. For example, pure water at 1 bar pressure will always freeze at 0oC and boil at 100oC. However, if impurities (solutes) are added to the ... Part 1 - Freezing of alcohol solutions
Solved 29. When one mole of benzene is vaporized at a ... (1 L-atm = 101.3 ) 30. Refer to the phase diagram for CO2, below a. Label the four regions of the phase diagram with the appropriate phase; Question: 29. When one mole of benzene is vaporized at a constant pressure of 1.00 atm and at its boiling point of 353.0 K, 30.79 kJ of energy (heat) is absorbed and the volume change is +28.90 L.
Isopropyl alcohol (data page) - Wikipedia Phase behavior Triple point: 184.9 K (−88.2 °C), ? Pa Critical point: 508.7 K (235.6 °C), 5370 kPa Std enthalpy change of fusion, Δ fus H o: 5.28 kJ/mol Std entropy change of fusion, Δ fus S o: 28.6 J/(mol·K) Std enthalpy change of vaporization, Δ vap H o: 44.0 kJ/mol Std entropy change of vaporization, Δ vap S o: 124 J/(mol·K) Solid ...
Solved Module 05 Content Question 1 1 Point Describe how ... А Alcohol B Mercury thing Water D Glycerin Using the phase diagram, identify the phase that water is in for the following conditions: 0.4kPa and -10°C D Critical point 22,089 Ice (solid) Water (liquid) Pressure (kPa) 101 - Triple point 0.6 1 B Water vapor (gas) А I 0 0.01 100 374 Temperature (°C) А Solid B Liquid Gas D Triple point E ...
Isopropyl Alcohol - NIST , The heats capacities of isopropyl alcohol and acetone from 16 to 298 °K and the corresponding entropies and free energies, J. Am. Chem. Soc., 1929, 51, 1145-1150. [ all data ] Domalski and Hearing, 1996
Chapter Review - General Physics Using Calculus I 1.5 Phase Changes. A pressure cooker contains water and steam in equilibrium at a pressure greater than atmospheric pressure. How does this greater pressure increase cooking speed? As shown below, which is the phase diagram for carbon dioxide, what is the vapor pressure of solid carbon dioxide (dry ice) at [latex]-{78.5}^\circ \text{C}[/latex]?
List of Phase Changes Between States of Matter - ThoughtCo Matter undergoes phase changes or phase transitions from one state of matter to another. Below is a complete list of the names of these phase changes. The most commonly known phase changes are those six between solids, liquids, and gasses. However, plasma also is a state of matter, so a complete list requires all eight total phase changes.
Isopropyl Alcohol - NIST Isopropyl Alcohol. Formula: C 3 H 8 O. Molecular weight: 60.0950. IUPAC Standard InChI: InChI=1S/C3H8O/c1-3 (2)4/h3-4H,1-2H3. Copy Sheet of paper on top of another sheet. IUPAC Standard InChIKey: KFZMGEQAYNKOFK-UHFFFAOYSA-N. Copy Sheet of paper on top of another sheet.
PDF the Energy of Evaporation | A Lab Investigation evaporation is a phase change that absorbs energy. During evaporation, some fast-moving, highly energetic molecules have enough energy to over-come the attractions that individual molecules have for one another and enter the gas phase. As these high-energy molecules leave the liquid phase, the average energy of the remaining liquid
phase change study guide.doc - Name:_ Date:_ Period ... The change from liquid water to steam (a gas) is a change in phase and requires the gain of heat energy. This energy can be gained (taken in) from the environment. When you put rubbing alcohol on your skin, it makes your skin feel cold.


(259).jpg)















0 Response to "39 phase change diagram for rubbing alcohol"
Post a Comment