39 molecular orbital diagram for he2+
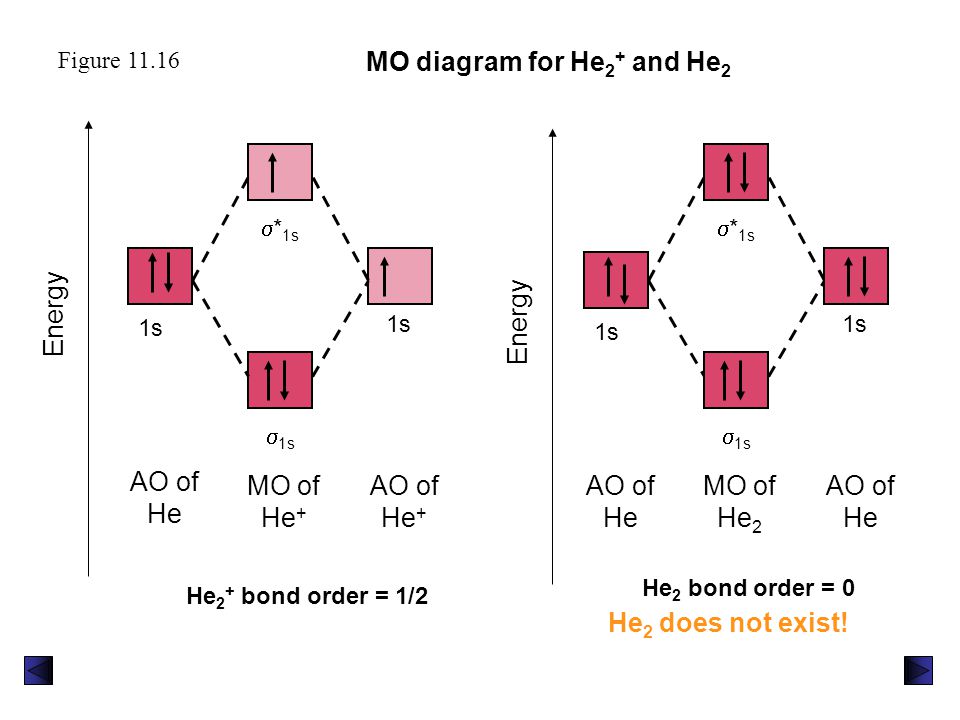
Why does He2 not exist? - Quora Answer (1 of 26): Helium and the other inert gases have completely filled octets, and so they have zero valency. That means, their combining capacity is zero, and so they exist as monatomic molecules such as He, Ne, Ar etc. Helium atom has the electronic configuration 1s2. According to the molec... Molecular Orbital Diagram For He2 A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. According to the molecular orbital theory, in a supposed He2 molecule, both the if we draw its MOT DIAGRAM, 2 e's enter the Bonding molecular Orbital and 2 .
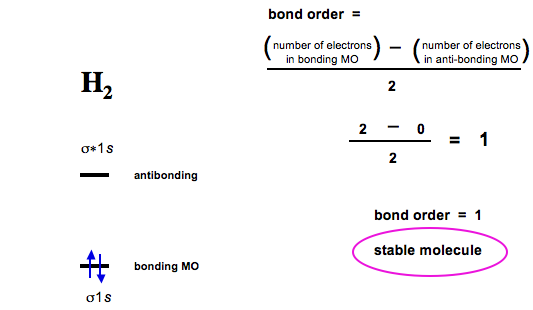
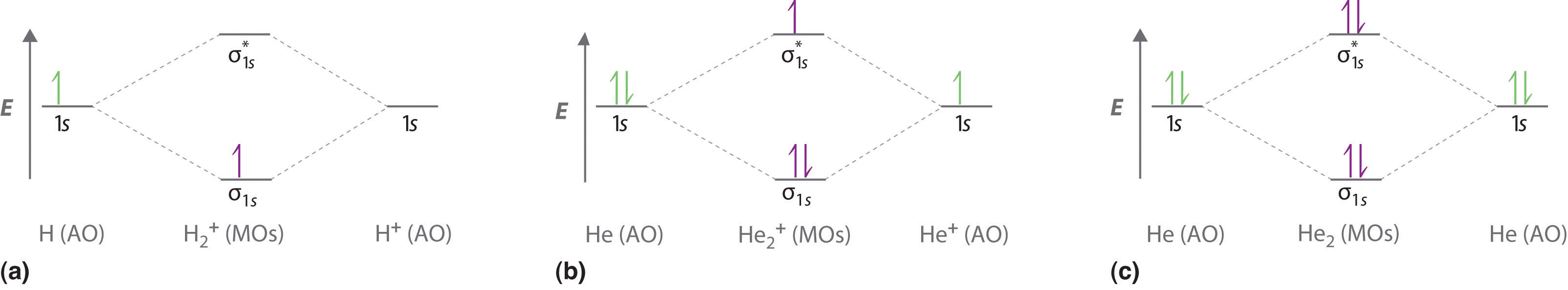
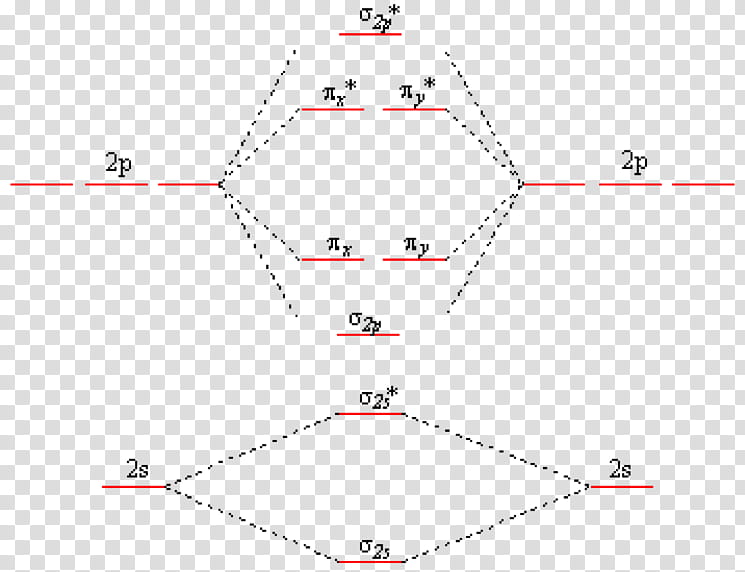
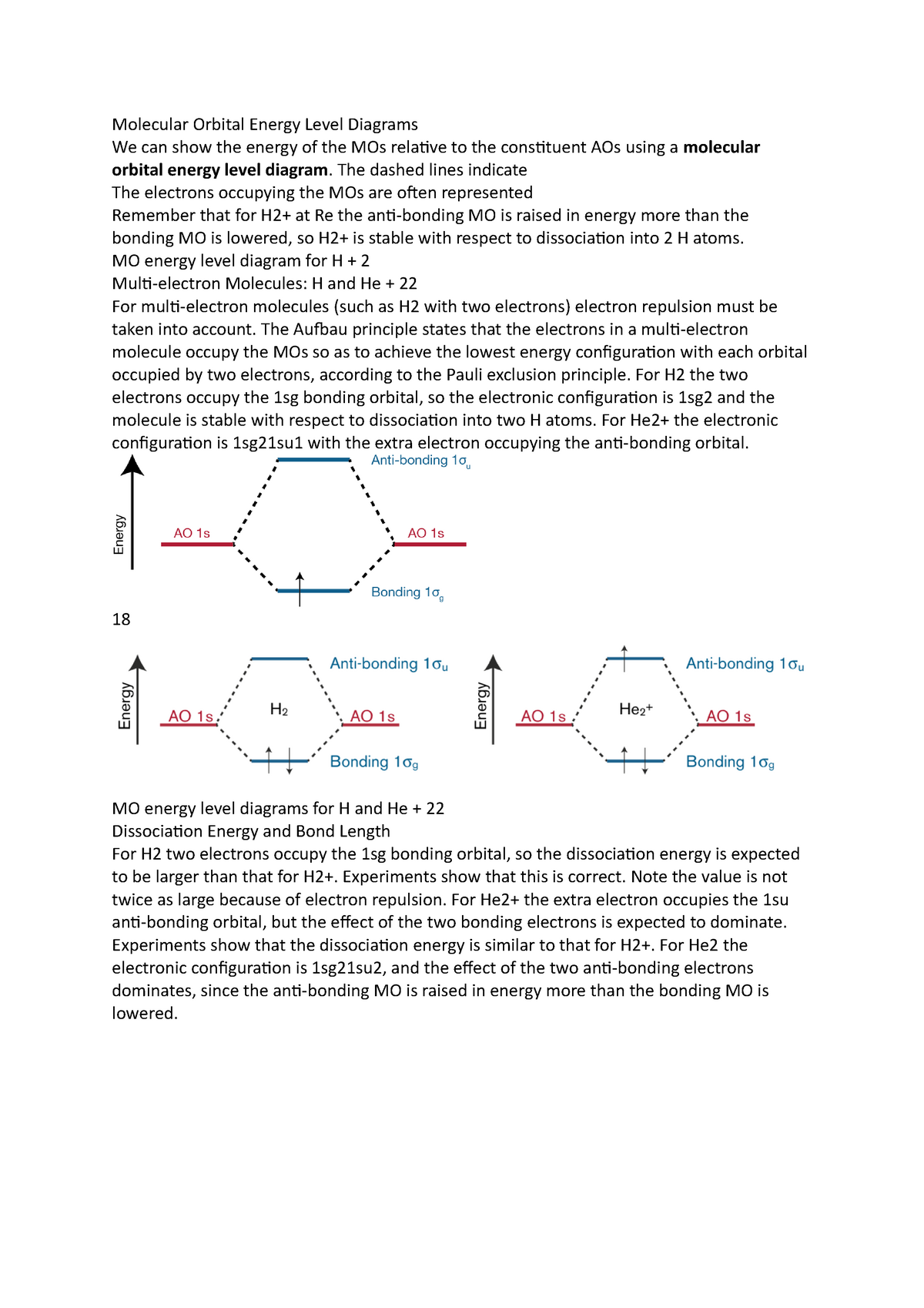
7.7 Molecular Orbital Theory - Chemistry Fundamentals A molecular orbital can hold two electrons, so both electrons in the H 2 molecule are in the [latex]\sigma[/latex] 1s bonding orbital; the electron configuration is [latex]{\left({\sigma}_{1s}\right)}^{2}.[/latex] We represent this configuration by a molecular orbital energy diagram (Figure 7.7.10) in which a single upward arrow indicates one ...
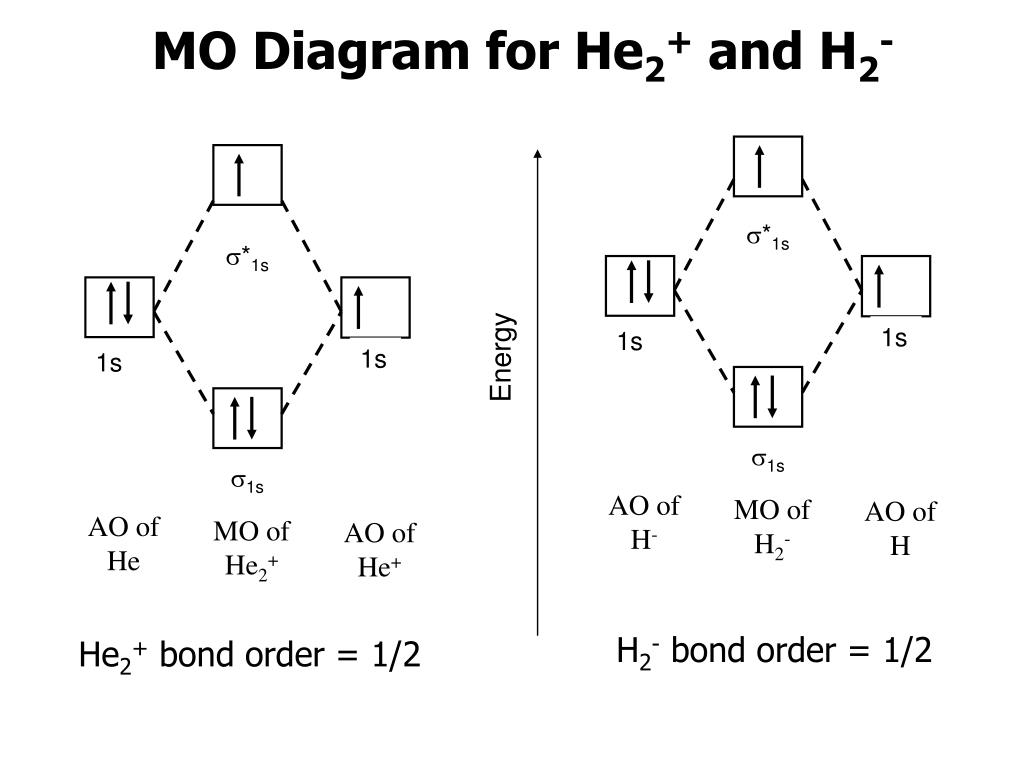
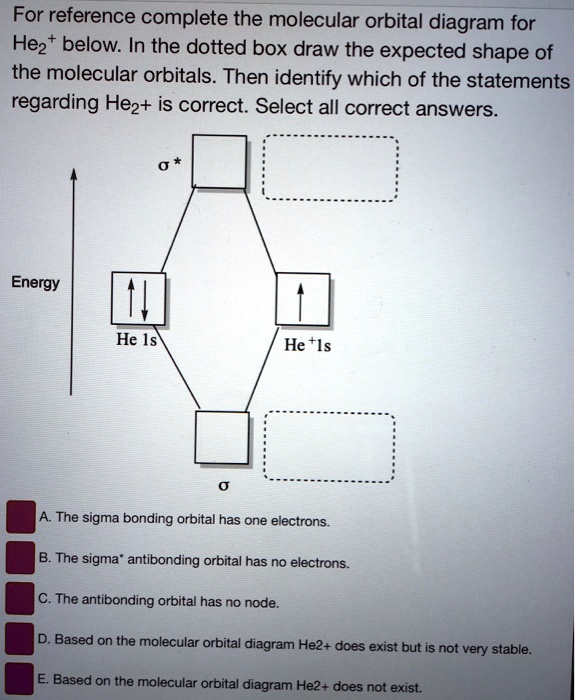
Molecular orbital diagram for he2+
What is the MOED of He2 molecule class 11 chemistry CBSE B o n d o r d e r = 1 2 [ N b − N a] And we got from the diagram that Helium has two electrons in its bonding molecular orbital and two electrons in its anti-bonding molecular orbital. So its bond order will be: B o n d o r d e r = 1 2 [ 2 − 2] = 0. So, the bond comes out to be zero, therefore the H e 2 molecule is unstable and does not exist. Molecular Orbital Diagram For He2 2+ - Wiring Diagrams Molecular Orbital Diagram For He2 2+ Two electrons total, both occupy the sigma orbital, two more electrons in bonding than antibonding He2 is not possible. Please note the diagram is for He2+ but the He-H is very similar answered Mar 21 '13 at A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and ... Molecular Orbital Theory - Purdue University Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
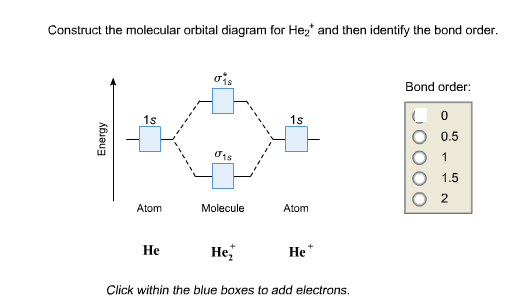
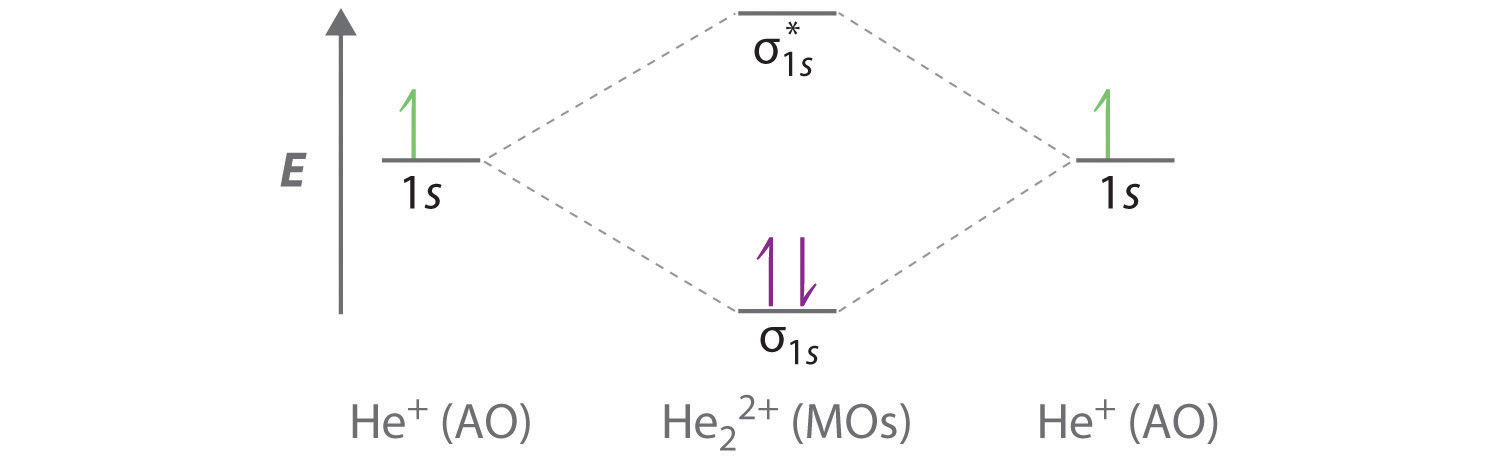
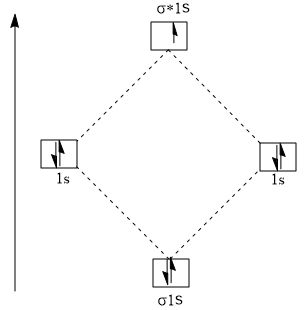
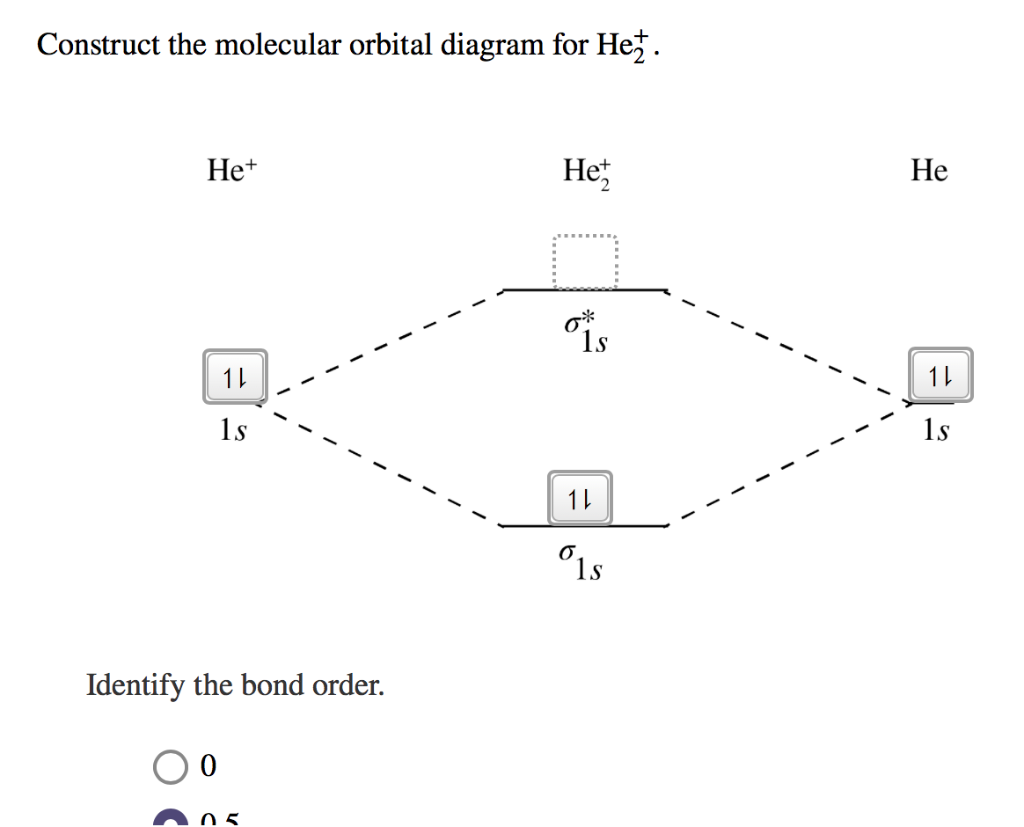
Molecular orbital diagram for he2+. Molecular Orbital Diagram For He2+ - schematron.org Molecular Orbital Diagram For He2+. He2+ MO diagram. Eg: Li + H; Li has 1s + 2s, while H has 1s. This mix to form a sigma orbital from H1s+Li2s, a sigma* orbital and H1s-Li2s. The bond order of a simple molecule can be determined by looking at the number of electrons in bonding and antibonding molecular orbitals. Like electrons in. Molecular Orbital Theory | Boundless Chemistry Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the energetically-favored configuration. Construct the molecular orbital diagram for He2+ Use molecular orbital theory, write a molecular orbital diagram, calculate bond order, and predict whether or not each ion exists in a relatively stable form, for the following two molecular ions for He2^2+ He2 2+ Molecular Orbital Diagram - Wiring Diagrams The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H2 molecule is shown in Figure Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe.
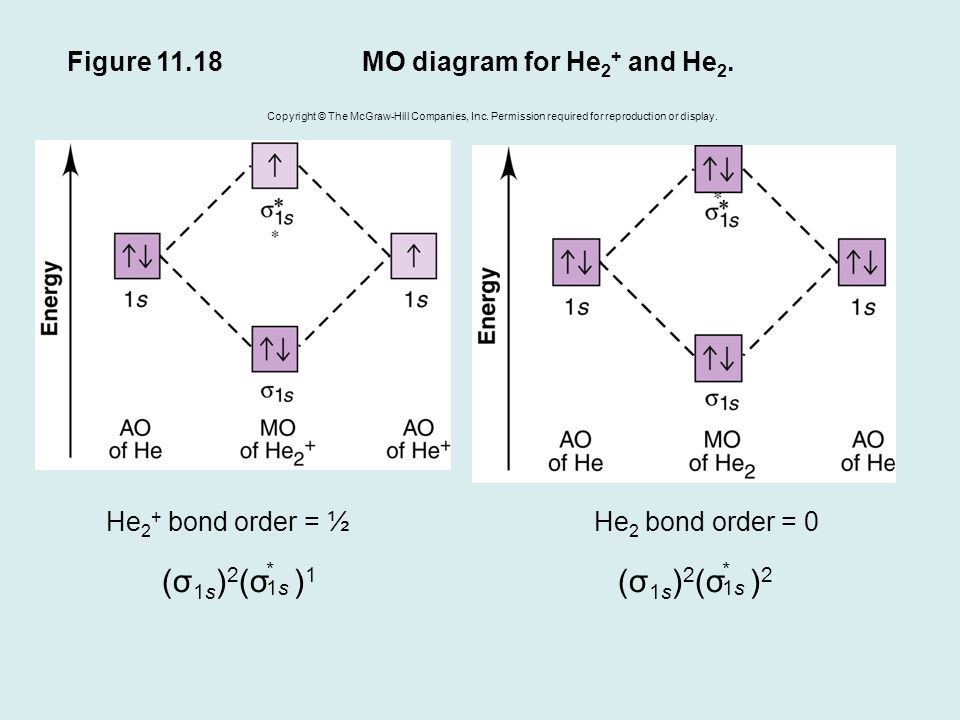
He2 2+ Molecular Orbital Diagram - schematron.org A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ... Do He2, He2(+), He2(2+) exist, stable? (Molecular Orbital ... In He2 (dihelium), the two 1s atomic orbitals overlap to create two molecular orbitals: sigma(1s) and sigma(1s)*. You fill these molecular orbitals with the... Construct the molecular orbital diagram for he2 - Soetrust Use the molecular orbital diagram shown to determine which… using the molecular orbital theory, describe the bonding in… Write orbital diagram for Au+? Decide if N2 and N2+ are paramagnetic or diamagnetic. Which… What is the bond order of C2 2- ? can someone please explain why SeF4's hybridization is sp3d? Molecular orbital correlation diagrams for He2, He2+, N2 ... After a preliminary check with He2 and He2+, self‐consistent field calculations have been carried out for the nitrogen and carbon monoxide molecules and some of their positive ions for the range of internuclear distances from about 1.5 times equilibrium down to 0.01 bohr. To adequately describe the passage from the separated to the united atom limits, basis sets comprised of even‐tempered ...
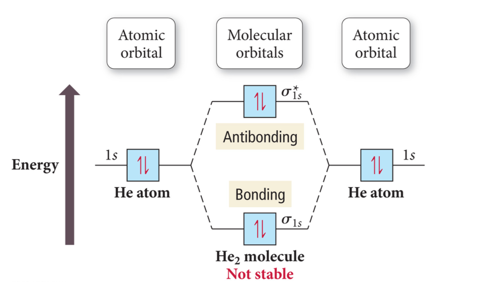
Why He2 molecule does not exist? Explain by MOT. Solution. Verified by Toppr. Electronic configuration of He is 1s 2. Molecular Orbital Diagram for He 2. . is. (Refer to Image) Bond order= 2(No. of electrons in bonding molecular orbital)- (No. of electrons in anti-bonding Molecular orbital) . Mo Diagram He2 And so, the. Molecular orbital theory (MO theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. It also explains.Show transcribed image text Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxes to add electrons. Solved Construct the molecular orbital diagram for He2 and ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 80% (83 ratings) Transcribed image text: Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxes to add electrons. chemical bonding - Molecular orbitals of H2 and He2 ... The molecular orbital energy-level diagram shown in Figure 13 also applies (with changes of detail in the energies of the molecular orbitals) to the hypothetical species He 2. However, this species has four valence electrons, and its configuration would be 1σ 2 2σ 2. Although there is a bonding influence from the two bonding electrons, there ...
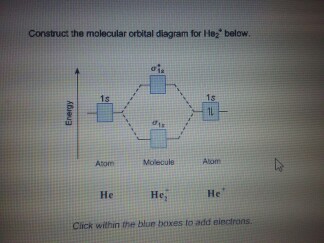
Construct the molecular orbital diagram for he2 Molecular orbital diagram has been drawn for the given molecule. This has totally 4 electrons in it. In molecular orbital diagram, it is clearly shown that the bonding orbital and the antibonding orbitals has two electrons each. Therefore, the number of bonding electrons are 2 and the number of anti-bonding electrons are 2.
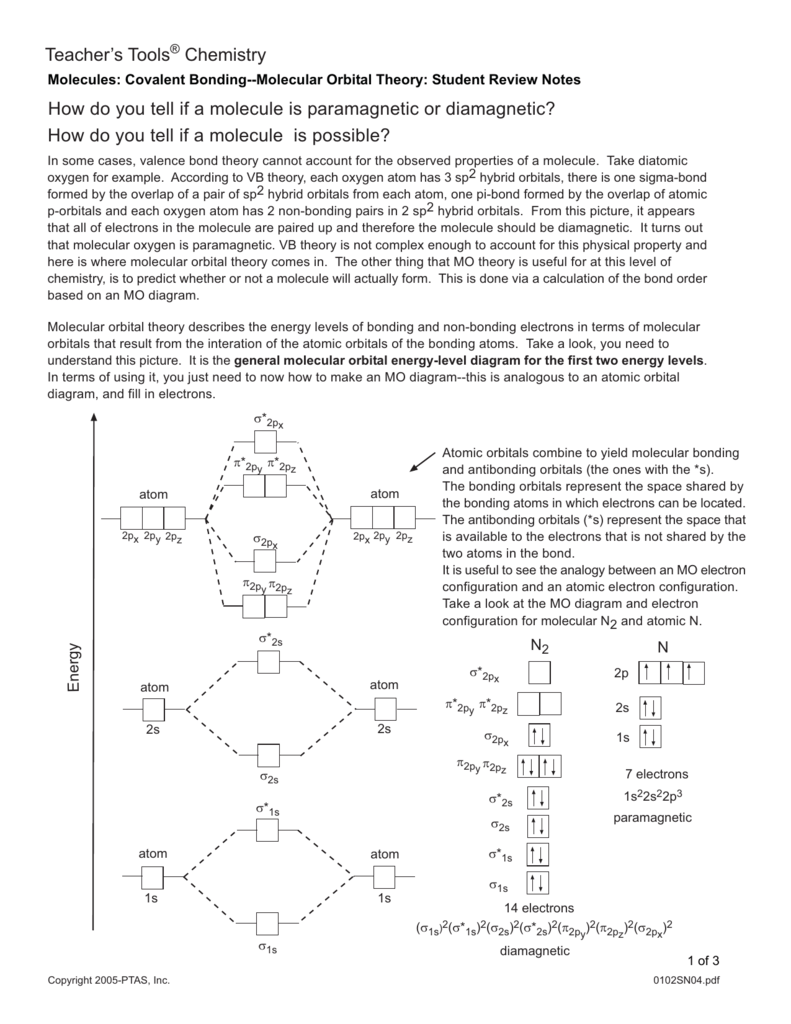
Molecular Orbital Theory The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule. Two are placed in the bonding orbital, the other two in antibonding orbital. The bond order = 1/2 x (Number of Bonding Electrons - Number of Antibonding Electrons) = 1/2 x (2-2)=0.
OneClass: construct the molecular orbital diagram for He2 ... construct the molecular orbital diagram for He2+2 and Sapling Learning Map d mcanoe Construct the molecular orbital diagram for Hez and then identify the bond order Bond order: 1s 1s D0.5 Ï . 1s 0 1.5 Atom Molecule Atom HeHe He Click within the blue boxes to add electrons O Next, Exit-
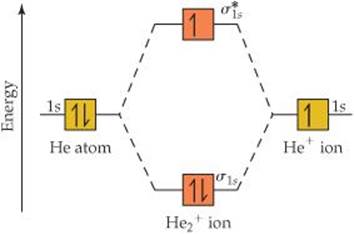
He2 - CHEMISTRY COMMUNITY There is a note in the course reader that says He2 is unstable, why is this? When I completed the molecular orbital diagram all the orbitals are filled and it is diamagnetic. Top. Hannah_1C Posts: 5 Joined: Fri Sep 20, 2013 10:00 am. Re: He2. Post by Hannah_1C » Wed Jul 27, 2016 8:16 am .
40 molecular orbital diagram for he2+ - Diagram For You The antibonding orbital is empty. Thus, H2 is a stable molecule. Construct the molecular orbital diagram for he2 Molecular orbital diagram has been drawn for the given molecule. This has totally 4 electrons in it. In molecular orbital diagram, it is clearly shown that the bonding orbital and the antibonding orbitals has two electrons each.
Why is He2 not a stable molecule? - CHEMISTRY COMMUNITY To answer the question, you must construct a molecular orbital (MO) diagram for the hypothetical He 2 molecule. The σ 1s bonding and antibonding orbitals will be full. Calculating the bond order results in 0. In other words, no bond can be sustained between two He atoms according to MO theory. Top.
Molecular orbital diarams for He2 and He2+ - YouTube How to write simple Molecular Orbital Diagrams and determine the Bond order
Molecular Orbital Theory - Purdue University Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
Molecular Orbital Diagram For He2 2+ - Wiring Diagrams Molecular Orbital Diagram For He2 2+ Two electrons total, both occupy the sigma orbital, two more electrons in bonding than antibonding He2 is not possible. Please note the diagram is for He2+ but the He-H is very similar answered Mar 21 '13 at A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and ...
What is the MOED of He2 molecule class 11 chemistry CBSE B o n d o r d e r = 1 2 [ N b − N a] And we got from the diagram that Helium has two electrons in its bonding molecular orbital and two electrons in its anti-bonding molecular orbital. So its bond order will be: B o n d o r d e r = 1 2 [ 2 − 2] = 0. So, the bond comes out to be zero, therefore the H e 2 molecule is unstable and does not exist.
















0 Response to "39 molecular orbital diagram for he2+"
Post a Comment