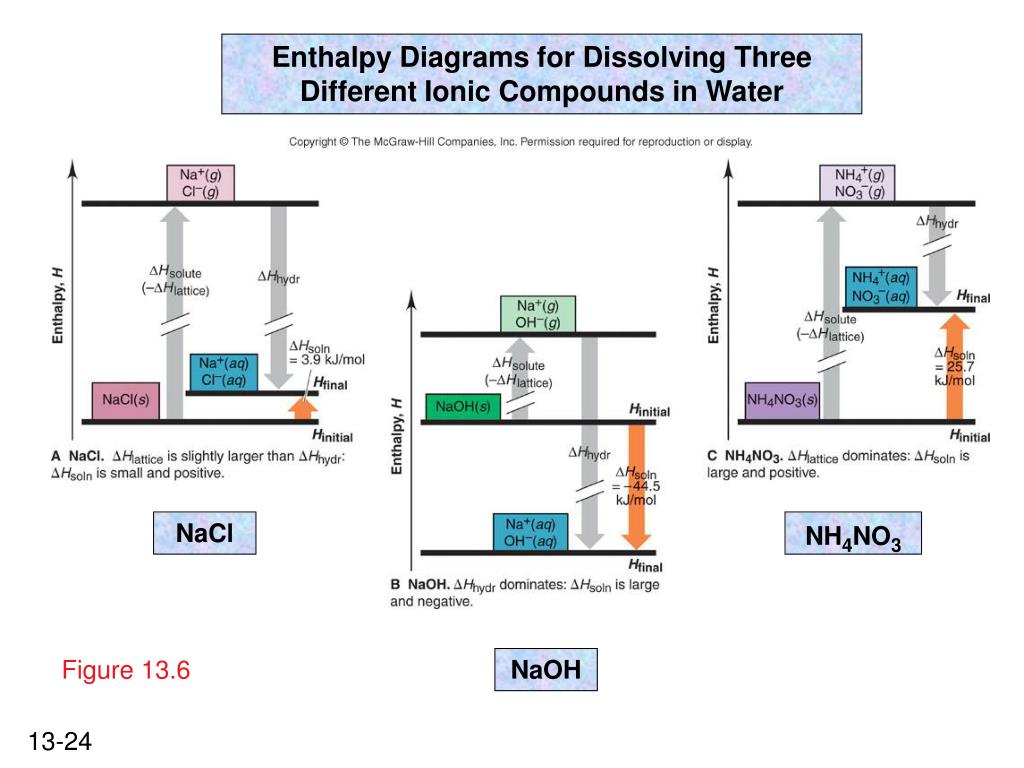
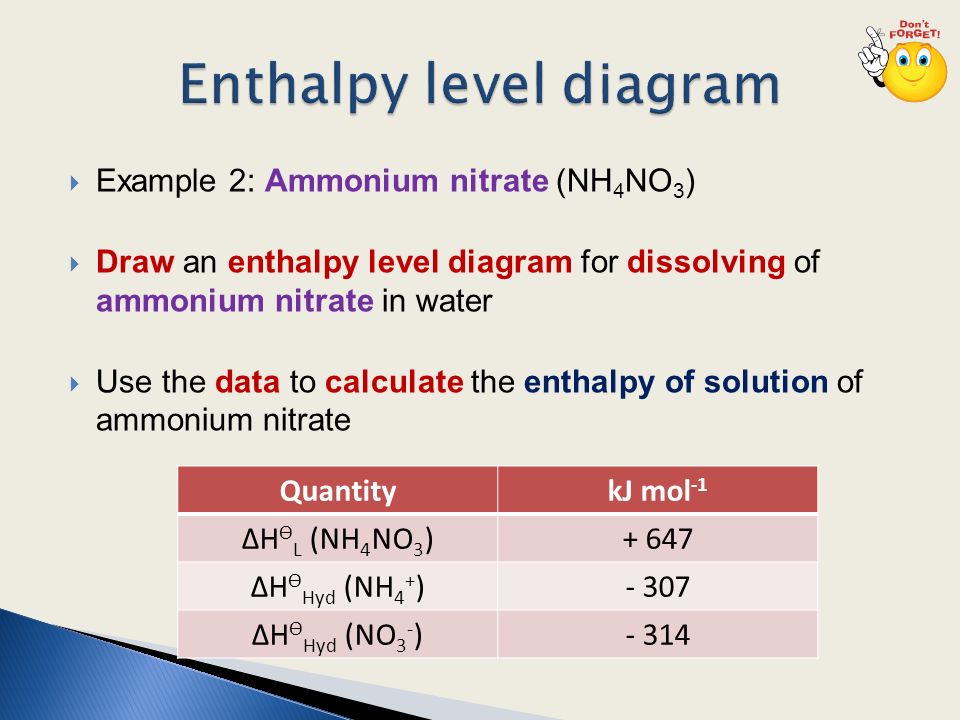
38 complete the enthalpy diagram for an ionic compound dissolving in water
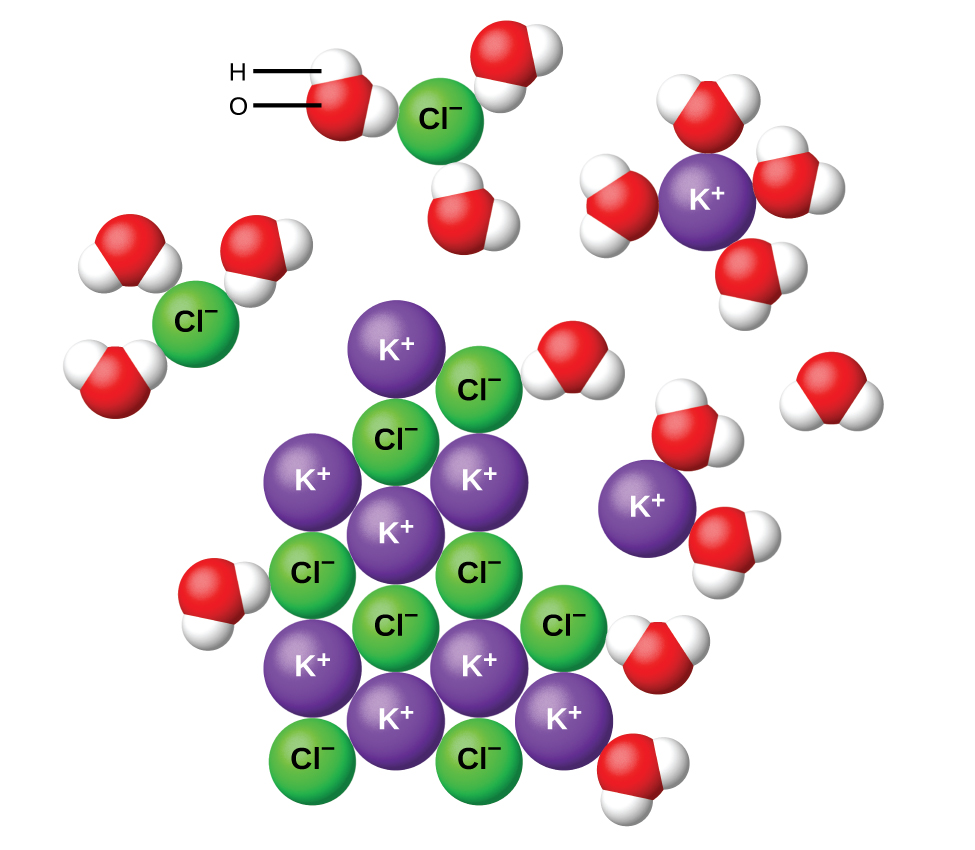
PDF (3) CE = 0 if O2− or water ionic or H bonding 1 (f) Magnesium oxide reacts with water / forms Mg(OH) 2 Allow MgO does not dissolve in water / sparingly soluble / insoluble 1 [11] Q3. (a) Enthalpy change for the formation of 1 mol of gaseous atoms allow heat energy change for enthalpy change 1 From the element (in its standard state) Water molecules and their interaction with salt | U.S ... Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
Solved Complete the enthalpy diagram for an Ionic compound Transcribed image text: Complete the enthalpy diagram for an Ionic compound dissolving in water 14 (80045.22 A'lg) X-(g) endothermic Enthalpy, ...
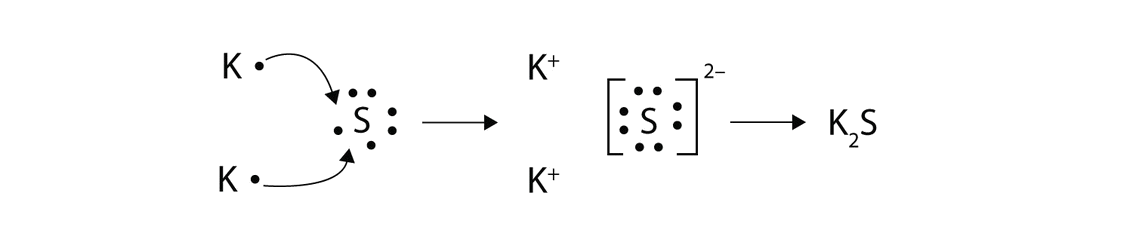
Complete the enthalpy diagram for an ionic compound dissolving in water
Enthalpy Lab - Semester2 - Copy.docx - SCH4U0 - Unit 1 Lab ... SCH4U0 - Unit 1 Lab: Determining the Enthalpy of Reaction In this lab, you will examine 2 reactions. The first reaction is between Magnesium Metal and Hydrochloric Acid, and the second reaction is the dissolution (dissolving) of the compound ammonium chloride. Using a coffee-cup calorimeter, you will conduct the reaction, examine the quantities of the reactants and stoichiometry, and then ... Dissolving Process | Chemistry for Non-Majors The Dissolving Process. Water typically dissolves many ionic compounds and polar molecules. Nonpolar molecules such as those found in grease or oil do not dissolve in water. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. Water molecules move about continuously due to ... Solved Complete the enthalpy diagram for an ionic compound Question: Complete the enthalpy diagram for an ionic compound dissolving in water B (g) Y-(g) vaporization Δ Hsolution B(aq) Y (aq) fusion final endothermic ...
Complete the enthalpy diagram for an ionic compound dissolving in water. Complete the enthalpy diagram for an ionic compound di… Complete the enthalpy diagram for an ionic compound dissolving in water B (g) Y-(g) vaporization Δ Hsolution B(aq) Y (aq) fusion final endothermic ... PDF Thermodynamics of Salt Dissolution - WebAssign Some ionic compounds dissolve readily in water, while others are insoluble. Some ionic com-pounds give o heat when dissolving in water and others absorb heat. Whether the dissolution process of a given ionic compound gives o or absorbs heat depends on the strength of the inter-molecular forces holding the solid together, as well as those ... Solved NAMI Served Complete the enthalpy diagram for an Question: NAMI Served Complete the enthalpy diagram for an Ionic compound dissolving in water. 14 OH solucion A'lg) X-(g) A formation endothermic AHsolute ... Complete the enthalpy diagram for an ionic compound ... The diagram represents the solution process (lattice energy, heat of hydration, and heat of solution) for an ionic compound dissolving in water. ht Arrow ...
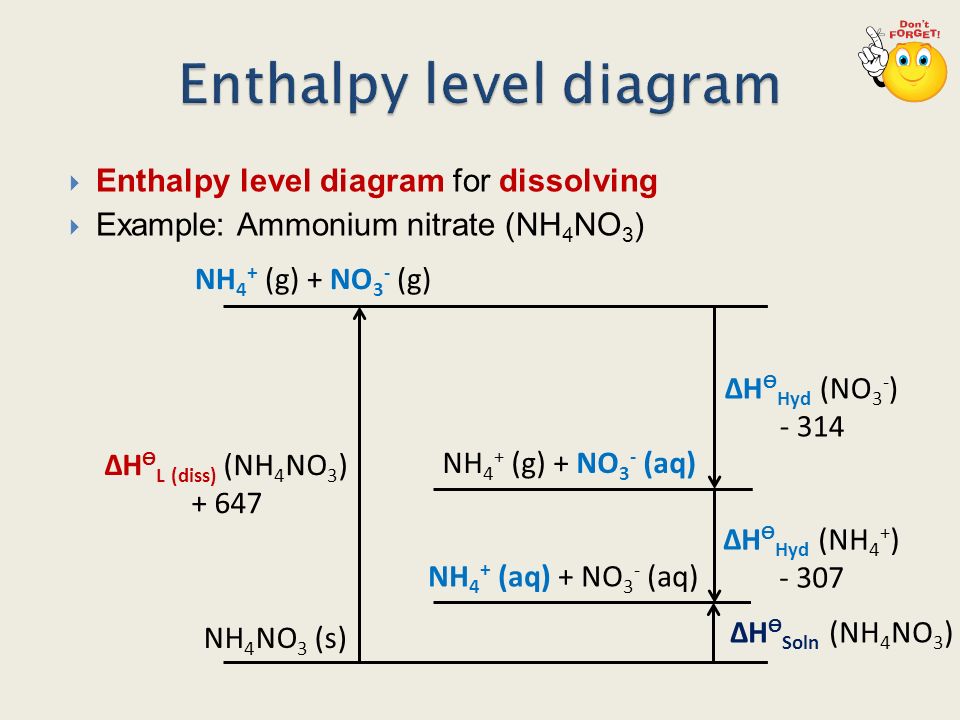
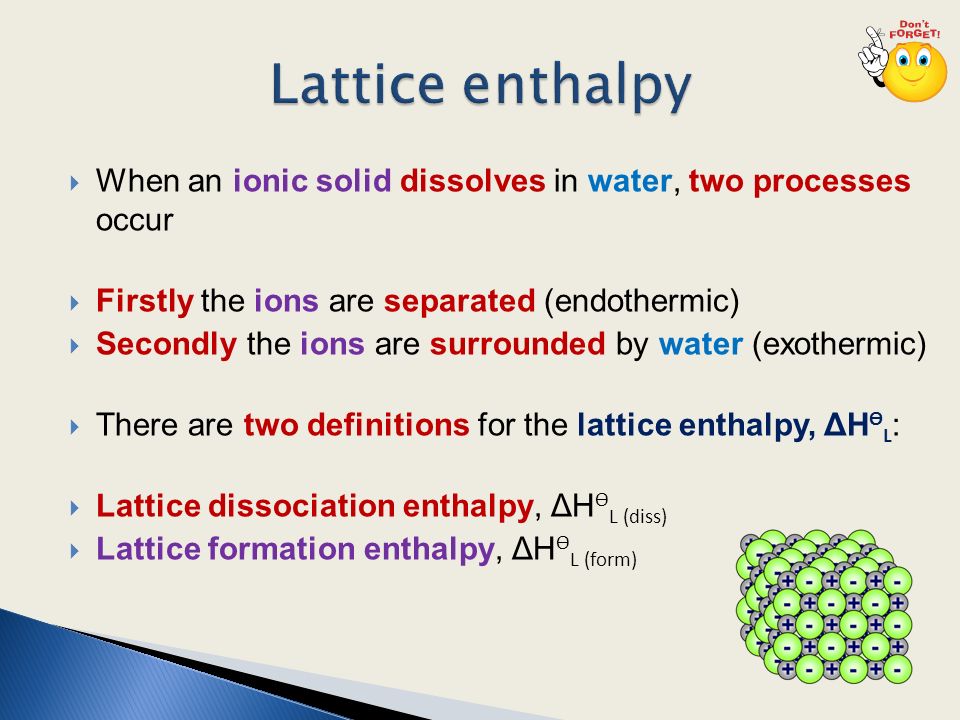
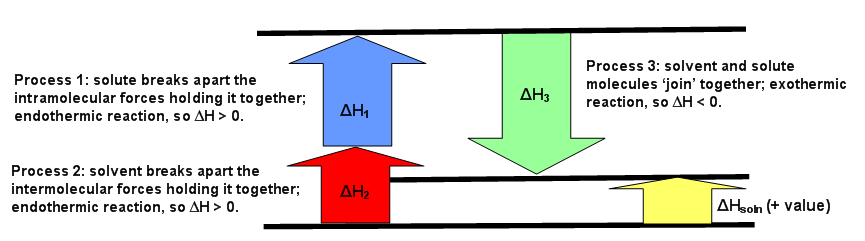
CHAPTER 2.0 THERMOCHEMISTRY_NOTES & TUTORIAL Q's - Flip ... When an ionic compound dissolves in water, positive end of water molecule will attract the negative ion & negative end of water will attract the positive ion DISSOLUTION OF IONIC SOLID INVOLVES 2 STEPS Step 1 Dissociation of lattice : Energy absorbed is the lattice dissociation energy MX(s) H2O M+ (g) + X− (g) Step 2 How to Draw & Label Enthalpy Diagrams - Video & Lesson ... An enthalpy diagram is a method used to keep track of the way energy moves during a reaction over a period of time. Learn how to draw and label enthalpy diagrams, the definition of an enthalpy ... Answered: Compound XY is an ionic compound that… | bartleby Compound XY is an ionic compound that dissociates as it dissolves in water. The lattice energy of XY is-591.8 kJ mol. The hydration energy of its ions is -627.8 kJ mol". Write the thermochemical equations for the two steps in the formation of a solution of XY in water. Draw an enthalpy diagram for the formation of this solution. PDF Lattice Enthalpy NEW - WordPress.com Enthalpy change of solution and Enthalpy change of Hydration When ionic compounds dissolve in water, there is usually a temperature change. Sometimes this is exothermic (e.g. dissolving calcium chloride) and sometimes endothermic (e.g. dissolving ammonium nitrate). The experiments are easy to carry out in a laboratory, and the usual equations ...
Solutions and Their Properties Flashcards | Quizlet It is the energy stored in the intermolecular attractions that hold particles together in an ionic solid. It is the energy of a solute. It is the amount of enthalpy change that occurs when 1mol of ionic solid is converted into gaseous ions. An ionic solid has lattice energy of 6473 kJ/mol and a hydration enthalpy of −6443 kJ/mol. Connect Assignment: Chapter 11 Flashcards | Quizlet PLAY. Match. Gravity. select all the options that represent the intermolecular forces broken or formed when NaCl is dissolved in water. Click card to see definition 👆. Tap card to see definition 👆. - some hydrogen bonding and dipole-dipole interactions in water are broken. - ionic bonds are broken in the solute. OneClass: complete the enthalpy diagram for an ionic compound ... Dec 11, 2019 · complete the enthalpy diagram for an ionic compound dissolving in water. Answer. + 20. Watch. 1. Solved Complete the enthalpy diagram for an ionic compound ... Chemistry. Chemistry questions and answers. Complete the enthalpy diagram for an ionic compound dissolving in water B (g) Y (g) AHsolution B' (aq) Y- (aq) Ahydration Ht final exothermic H solute AHvaporization BY (S) Hinitial endothermic Hormation This reaction is.
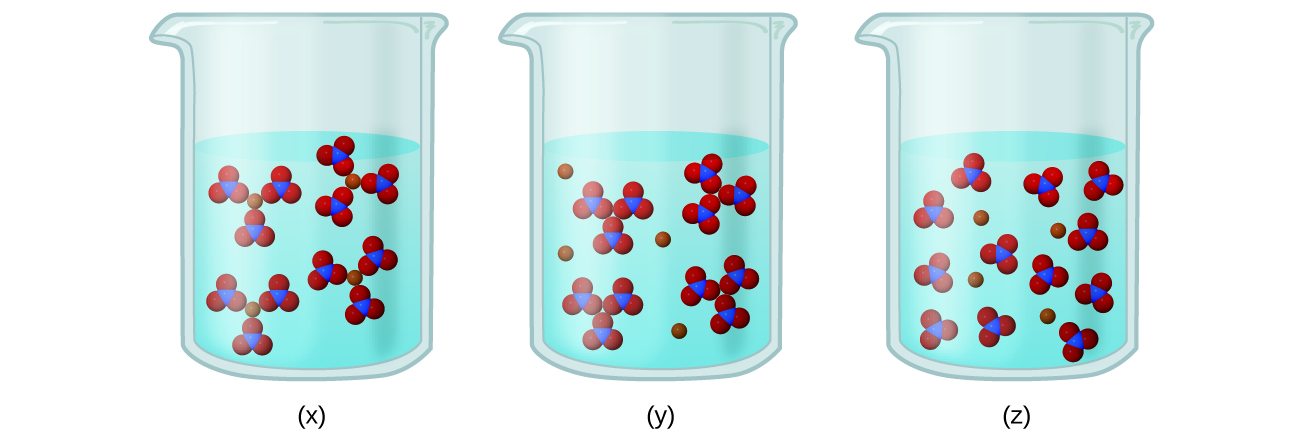
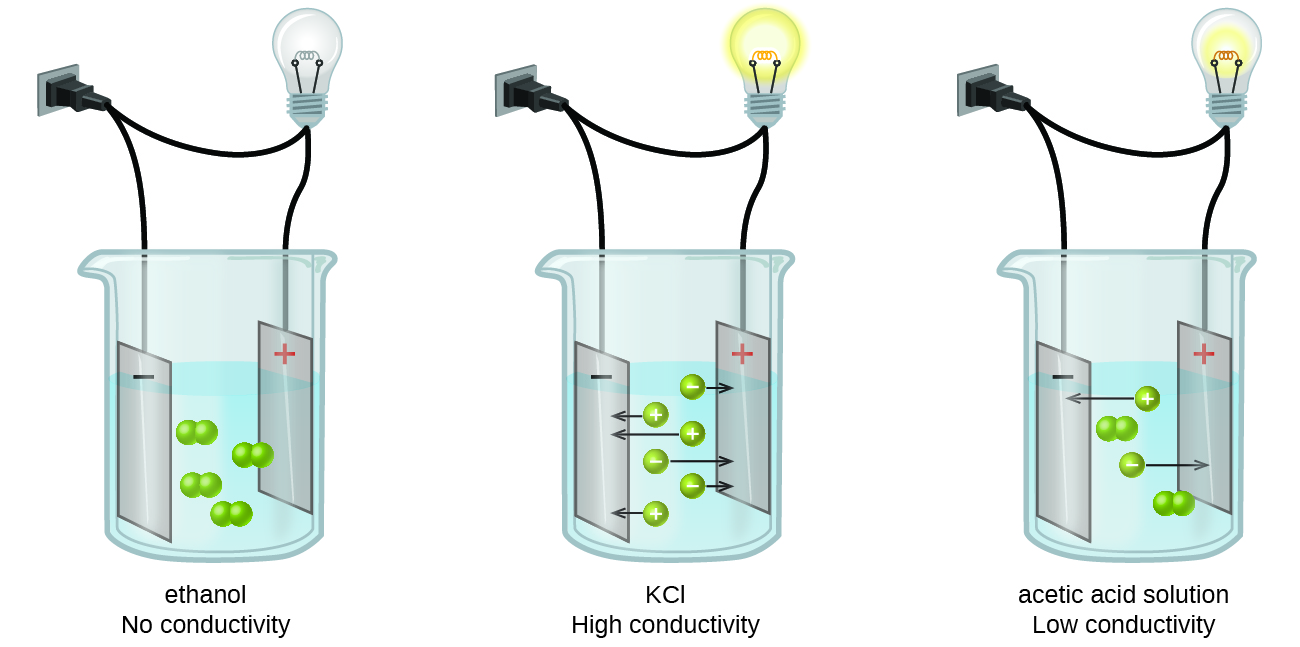
11.2 Electrolytes - Chemistry When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. These substances constitute an important class of compounds called electrolytes.Substances that do not yield ions when dissolved are called nonelectrolytes.If the physical or chemical process that generates the ions is essentially 100% efficient (all of the dissolved ...
Comparison between Covalent and Ionic Compounds ... At room temperature and normal atmospheric pressure, covalent compounds may exist as a solid, a liquid, or a gas, whereas ionic compounds exist only as solids. Although solid ionic compounds do not conduct electricity because there are no free mobile ions or electrons, ionic compounds dissolved in water make an electrically conductive solution.
Chemistry Chapter 7 Flashcards - Quizlet Terms in this set (70) Combustion Reactions. A reaction in which a substance reacts with oxygen, emitting heat and forming one or more oxygen-containing compounds. Evidence of a chemical reaction: -color change. -formation of a solid in a previously clear solution. -the formation of a gas when we add a substance to a solution.
Solved Complete the enthalpy diagram for an ionic compound ... Complete the enthalpy diagram for an ionic compound dissolving in water. A (g) X-(g) AHnydration AHusion exothermic AX(s) initial ??,aporization endothermic A (aq) x-(aq) A Hsolution Hrinal ??,ormation AHsolute This reaction is ; Question: Complete the enthalpy diagram for an ionic compound dissolving in water. A (g) X-(g) AHnydration AHusion ...
Solved Complete the enthalpy diagram for an ionic compound ... Chemistry. Chemistry questions and answers. Complete the enthalpy diagram for an ionic compound dissolving in water. exothermic B* (9) Y- (g) AHvaporization AHsolute B* (aq) Y- (aq) endothermic Enthalpy, H Hfinal Anydration Arusion BY (s) A Hormation Hinitial AH solution This reaction is.
Solved Complete the enthalpy diagram for an ionic compound ... Answer : The given is the complete enthalpy diagram for the ionic compound. The AX (s) is separated into its ion in gaseous forms that is A+ (g) and X- (g). This will require the solute. When the …. View the full answer. Transcribed image text: Complete the enthalpy diagram for an ionic compound dissolving in water.
Solubility - Purdue University We can generally assume that salts dissociate into their ions when they dissolve in water. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the energy required to separate the water molecules so that the ions can be inserted into solution.
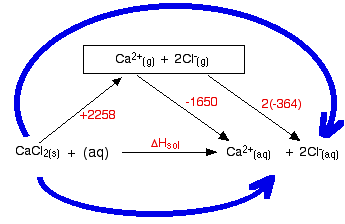
Cambridge International Examinations Cambridge ... - XtremePapers 1 (a) The dissolving of an ionic compound in water is accompanied by an energy change, the enthalpy change of solution, ∆H sol. MgCl 2+2(s) + aq → Mg (aq) + 2Cl –(aq) Describe, in terms of bond breaking and bond making, what happens to the solid ionic lattice when an ionic compound dissolves in water.
Lab 11 - Thermodynamics of Salt Dissolution Below is an example of a reaction for the dissolution of an ionic compound in water. NaCl ( s) → Na + ( aq) + Cl - ( aq ) When a soluble ionic compound is placed in water, the solid is converted to the product of the dissolution reaction—the solid vanishes, converting to dissolved ions in solution. 3
PDF FACTFILE: GCE CHEMISTRY - Council for the Curriculum ... Dissolving ionic compounds in water When an ionic compound dissolves in water two processes occur 1. Energy has to be taken in to break up the lattice and separate the positive and negative ions. This is the lattice enthalpy 2. The ions become surrounded by solvent and bonds form - energy is released when these ions form bonds with water molecules.
PDF Identifying and Comparing Properties of Ionic and Covalent ... 4. Based upon the compounds you researched, and your Venn diagram, are there any patterns with respect to the property of solubility? _____Yes_____ Explain Answers will vary but should include statements of like dissolves like. Ionic compounds will be water soluble and molecular compounds will not be water soluble. 5.
Unit-5-Chemistry-questions.doc - Name Unit 5 Chemistry ... Sodium chloride is an ionic compound that dissolves in water. The solution contains aqueous ions Na + (aq) and Cl - (aq). A concentrated aqueous solution of sodium chloride is known as brine. (a) Standard enthalpy changes of hydration can be used as part of an energy cycle to predict the solubility of an ionic compound, such as sodium ...
Why do ionic compounds dissolve in water? | Socratic Ionic compounds dissolve in water because the water molecules hydrate the ions. > To dissolve an ionic compound, the water molecules must be able to stabilize the ions that result from breaking the ionic bond. They do this by hydrating the ions. Water is a polar molecule. It has a permanent dipole. The "O" atom has a partial negative charge, and the "H" atoms have a partial positive charge.
CHM 1025C: Chapter 5 & 6 Review Flashcards - Quizlet The active ingredient in Milk of Magnesia™ is Mg (OH)2. Magnesium hydroxide is insoluble in water, so the product is a mixture of water, flavoring, and other ingredients to suspend the solid Mg (OH)2. When someone suffering from acid indigestion takes this drug, the Mg (OH)2 reacts with the HCl in the stomach.
Chemistry 1 Exam Flashcards - Quizlet Calculate the amount of heat required (in kilojoules) to heat 5.00 grams of water from -16.0 C to 11.0 c.-enthalpy of vaporization for water is 40.56 kJ/mol-enthalpy for fusion of water is 6.007 kJ/mol -specific heat for ice is 2.090 J/(gram x *C)-specific heat for water is 4.184 J/(gram x *C)-specific heat for steam is 2.030 J/(gram x *C)
Solved Complete the enthalpy diagram for an ionic compound Question: Complete the enthalpy diagram for an ionic compound dissolving in water B (g) Y-(g) vaporization Δ Hsolution B(aq) Y (aq) fusion final endothermic ...
Dissolving Process | Chemistry for Non-Majors The Dissolving Process. Water typically dissolves many ionic compounds and polar molecules. Nonpolar molecules such as those found in grease or oil do not dissolve in water. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. Water molecules move about continuously due to ...
Enthalpy Lab - Semester2 - Copy.docx - SCH4U0 - Unit 1 Lab ... SCH4U0 - Unit 1 Lab: Determining the Enthalpy of Reaction In this lab, you will examine 2 reactions. The first reaction is between Magnesium Metal and Hydrochloric Acid, and the second reaction is the dissolution (dissolving) of the compound ammonium chloride. Using a coffee-cup calorimeter, you will conduct the reaction, examine the quantities of the reactants and stoichiometry, and then ...



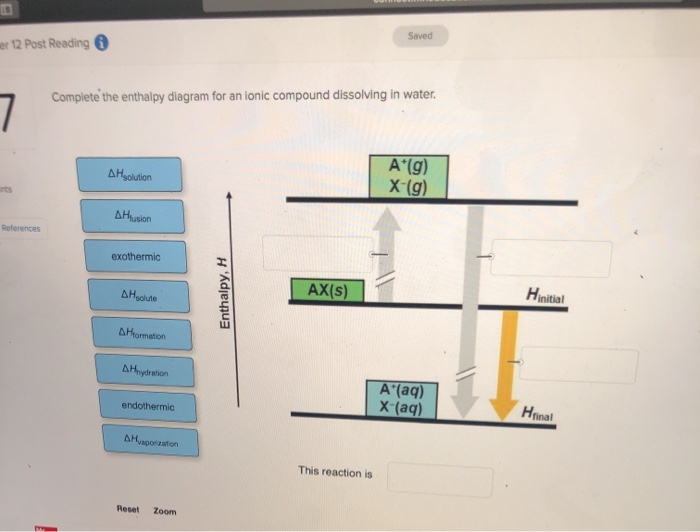
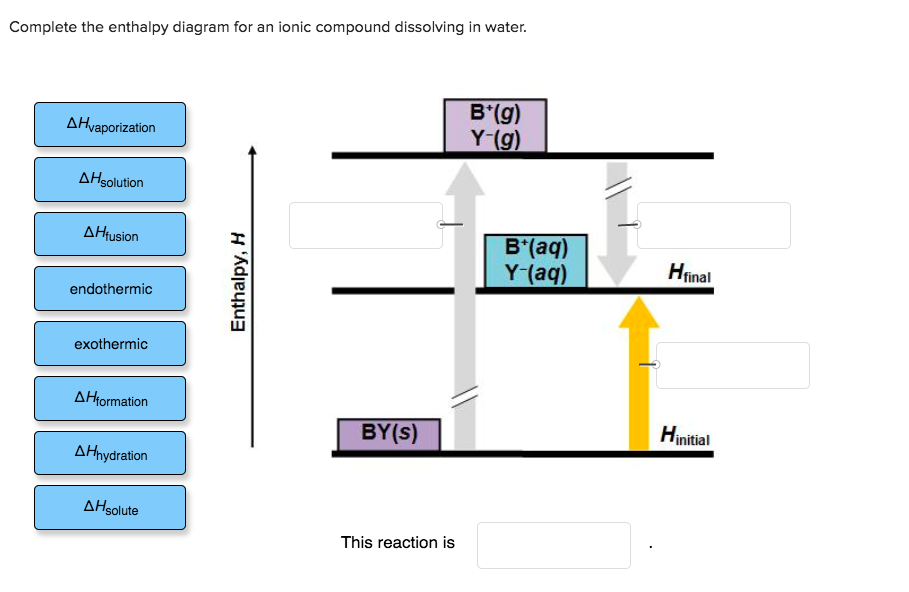

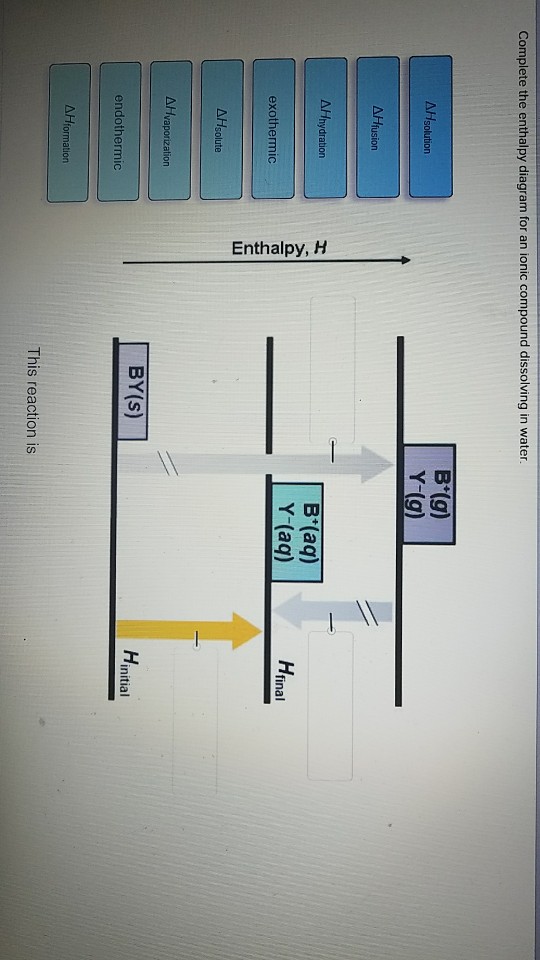
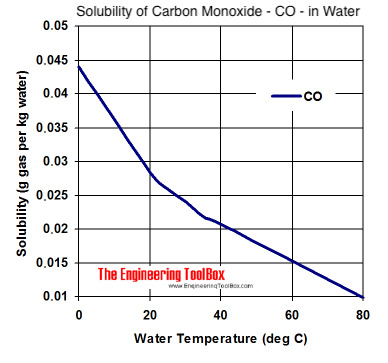


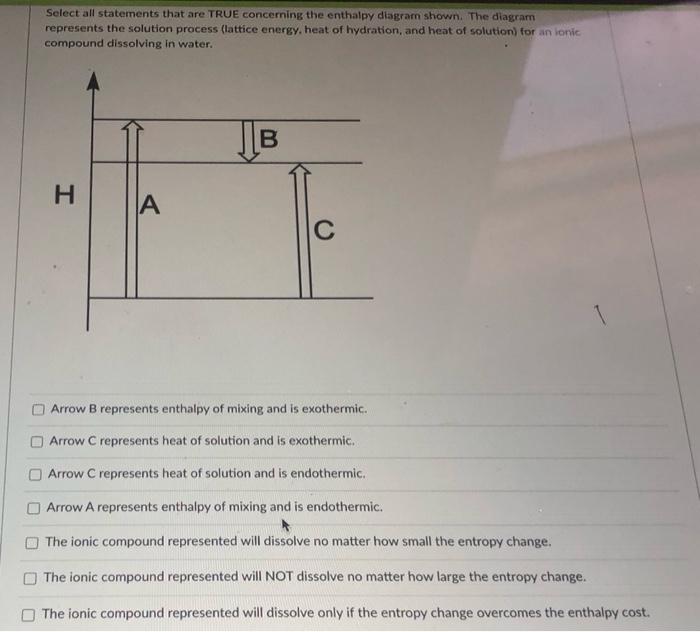











0 Response to "38 complete the enthalpy diagram for an ionic compound dissolving in water"
Post a Comment