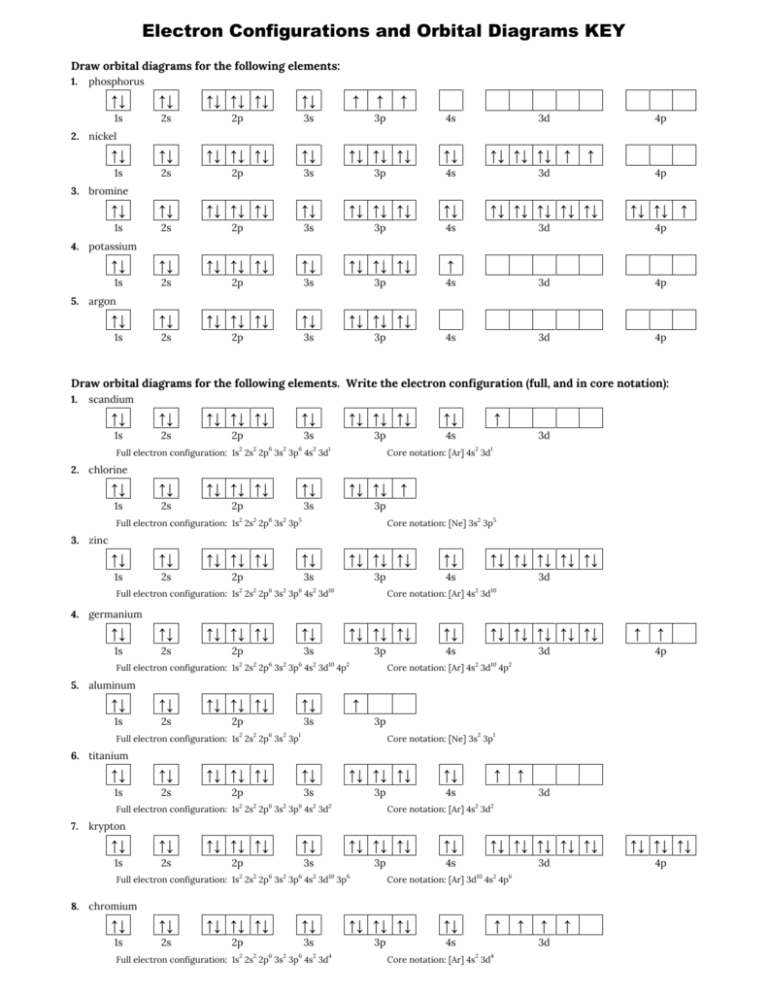
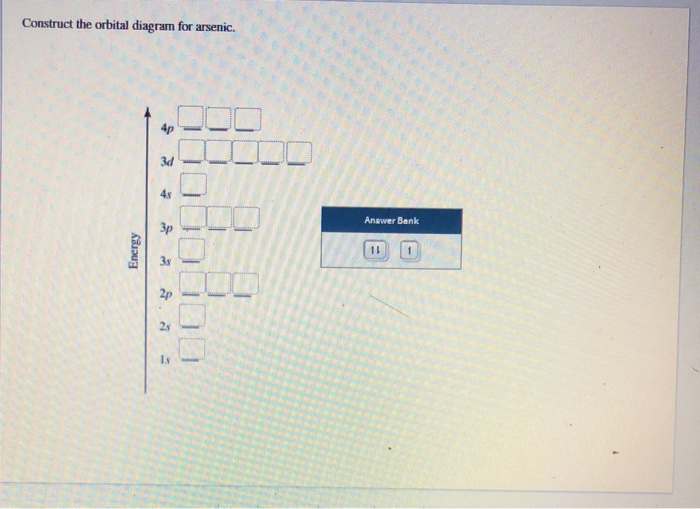
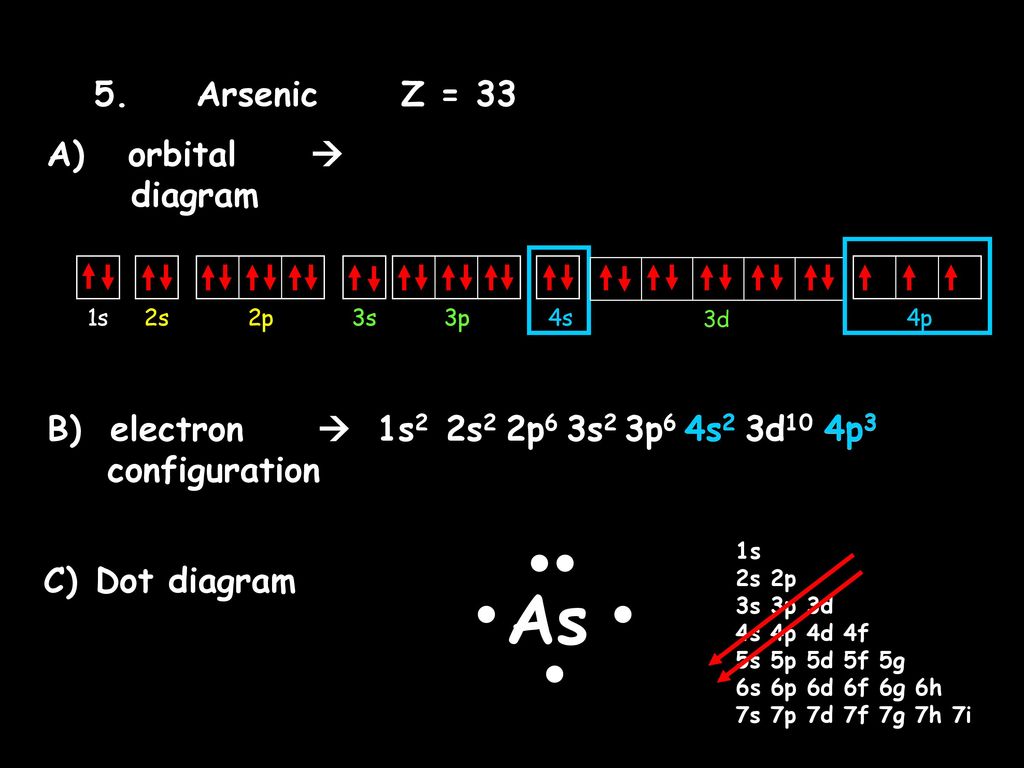
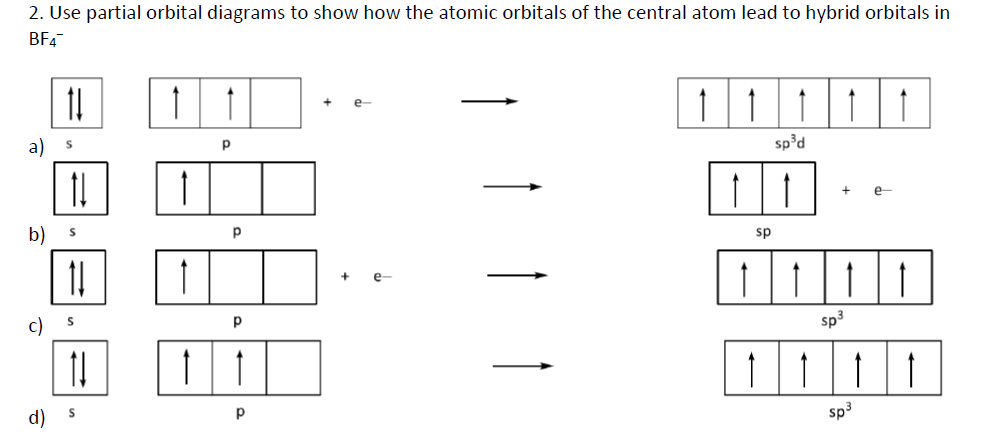
42 orbital diagram for as
The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in itsoutermost shell. schematron.org! Arsenic atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. An orbital box diagram can be written as well. Boxes, or horizontal lines represent the orbitals, arrows represent the electrons, and if an orbital is full, the electrons must be of opposite spin-one arrow pointing up and the other one pointing down. The orbital box diagrams are listed for the first 20 elements in the figure below.
Chemistry Orbital Diagrams Worksheet. Whether your children are being enlightened in the house by you or in a class setup, you can give them these activities as research. If you intend to utilize these in a class, have pupils compose their names, teacher names, section numbers, and the day before beginning the job available.
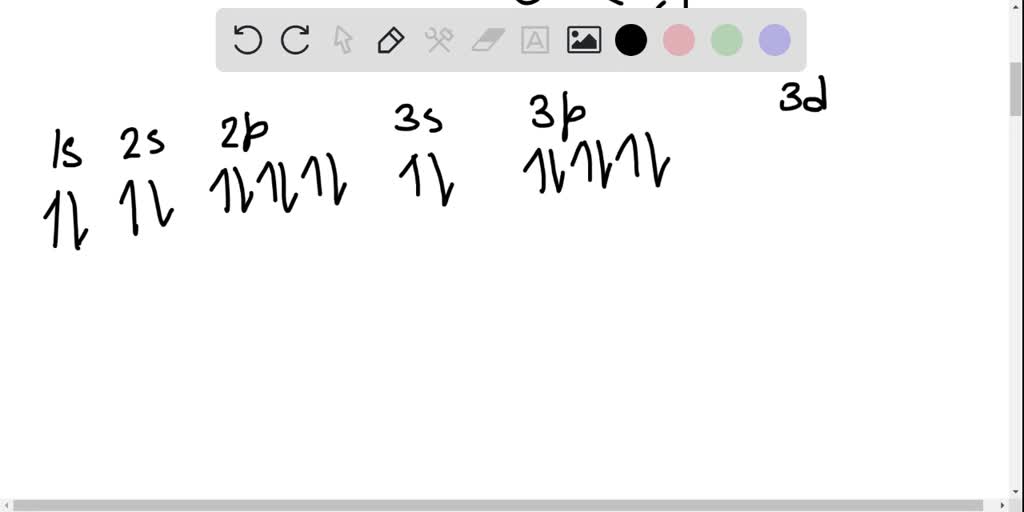
Orbital diagram for as
Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels. You’ll also notice that each module in a molecular orbital diagram has two sides and a middle section. Each of the two sides represent the atomic orbitals of each atom involved in the bonding, while the middle section represents the molecular orbital formed as the atomic orbitals combine. Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Orbital diagram for as. It is common to omit the core electrons from molecular orbital diagrams and configurations and include only the valence electrons. Figure 8. This is the molecular orbital diagram for the homonuclear diatomic Be 2 +, showing the molecular orbitals of the valence shell only. The molecular orbitals are filled in the same manner as atomic orbitals ... Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau... In picture 1 we show the molecular orbital structure of F2. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. The dashed lines show the remaining p orbitals which do not take part in the bonding. σ z y x σ* x y z Construct the molecular orbital diagram for ... First, we need to determine the electron configuration for As (arsenic). The electron configuration depends on the number of electrons an atom or ion has. Since As is neutral (uncharged), we can say that Z (atomic number) = number of protons = number of electrons. Arsenic has an atomic number of 33, so it has 33 electrons. 90% (339 ratings)
Table 8.3 Partial Orbital Diagrams and Electron Configurations * for the Elements in Period 4. * Colored type indicates the sublevel to which the last electron is added. 8-26. Figure 8.10 A periodic table of partial ground- state electron configurations. 8-27. Figure 8.11. Neon (Ne) electron configuration with full orbital diagram. Neon (Ne) is the tenth element in the periodic table and the 2nd element in group-18. The atomic number of neon is 10 and its symbol is 'Ne'. The standard atomic mass of neon is 20.1797 and it is an inert element. This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... Orbital Filling Diagrams An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. Enter a number for the the electrons that are paired or unpaired in each of the sublevels. Paired Unpaired. Mar 23 Show the orbital-filling diagram for sulfur.
Molecular-orbital diagrams as obtained by the ROHF method. Dashed lines indicate MOs dominated by the metal d-orbitals, the solid lines stand for doubly occupied or virtual ligand orbitals. Orbitals which are close in energy are presented as degenerate the average deviation from degeneracy is approximately 0.01 a.u. The order in which orbitals are listed on an orbital diagram follows: The Aufbau principal. Hund's rule states that the electron configuration with the lowest-energy will have the maximum possible number of unpaired electrons. Which of the elements below would require special attention to this rule to correctly depict the orbital diagram? An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s → 2s → 2p x 2p y 2p z → 3s → 3p x 3p y 3p z → The orbital diagram for hydrogen can be represented in the following way. This notation uses a box to represent the orbital, the label for the orbital and an arrow to represent the electron. The electronic configuration for hydrogen can be written as 1s 1. This is a short-hand notation which identifies the level, the sublevel and the number of ...
To write the orbital diagram of chlorine(Cl), you have to do the electron configuration of chlorine. Which has been discussed in detail above. 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise ...
Simple pictures showing orbital shapes are intended to describe the angular forms of regions in space where the electrons occupying the orbital are likely to be found. The diagrams cannot show the entire region where an electron can be found, since according to quantum mechanics there is a non-zero probability of finding the electron (almost ...
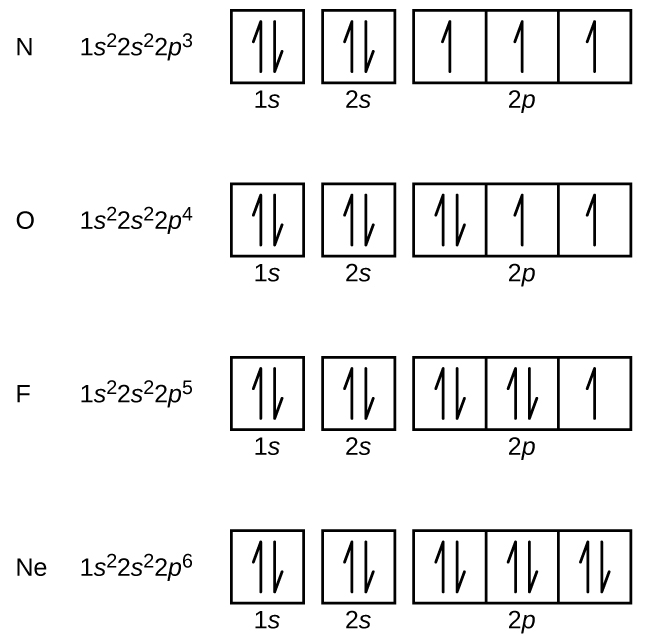
Orbital diagram of Nitrogen (N) 8. Orbital diagram of Oxygen (O) 9. Orbital diagram of Fluorine (F) 10. Orbital diagram of Neon (Ne) 11. Orbital diagram of Sodium (Na)
Orbital diagrams are a pictorial description of electrons in an atom. In order to figure out where electrons go in an atom we have to follow 3 main rules. The first one being the Auf Bau Principle, the Auf Bau Principle states that each electron occupies the lowest energy orbital available. Then we have to think okay with the sublevels, I mean ...
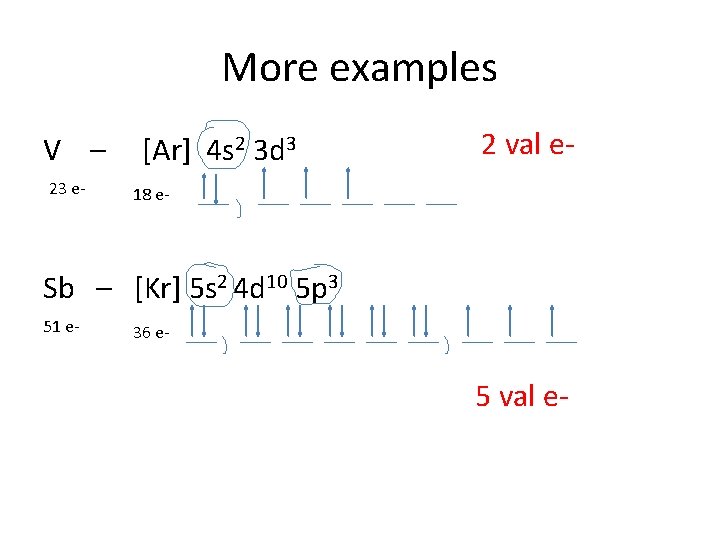
An orbital diagram is similar What is the orbital diagram for. For example, write the electron configuration of scandium, Sc: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1. So for scandium the 1 st and 2 nd electron must be in 1s orbital, the 3 rd and 4 th in the 2s, the 5 th through 10 th in the 2p orbitals, etc. 6/14/ Ch 8 4/18 Correct Part B Complete ...
Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution We draw a molecular orbital energy diagram similar to that shown in .
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of ...
Molecular Orbital Energy Diagrams. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right.
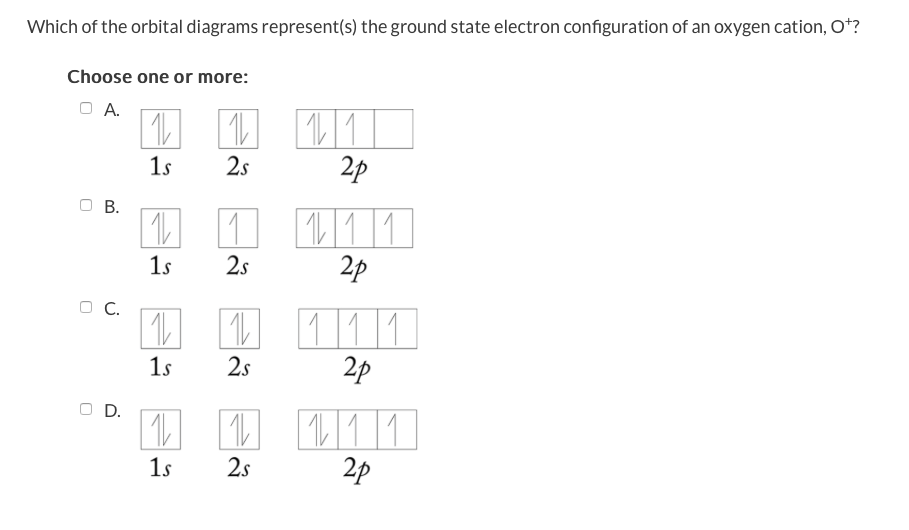
The electron configuration for oxygen is: 1s^2 2s^2 2p^4 This video will walk you through the step of writing orbital diagram. The video uses Kr as an example, but the process is exactly as the same as what you need to do for oxygen. Hope this helps!
Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal –each electron occupies the lowest energy orbital available; German for “build up” •Electrons are notated with an arrow –Up arrow goes first then, down arrow –Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
Molecular Orbital Diagrams of more complicated molecules XH 2 (D ∞ h) H--X--H molecular orbitals general MO diagram layout linear central atom's (X 's) atomic orbitals atomic orbitals of terminal atoms H 1 + H 2 a linear combination of symmetry adapted atomic orbitals (LGO's)
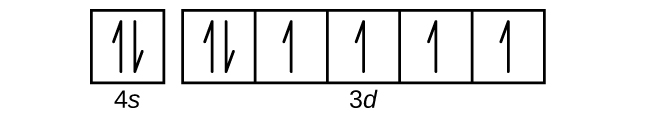
The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ...
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
You’ll also notice that each module in a molecular orbital diagram has two sides and a middle section. Each of the two sides represent the atomic orbitals of each atom involved in the bonding, while the middle section represents the molecular orbital formed as the atomic orbitals combine.
Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.
![Molecular orbital diagram of [CoF 6 ] 3-complex with six p ...](https://www.researchgate.net/profile/Majid-Monajjemi/publication/257140982/figure/tbl1/AS:669050661257229@1536525516648/Molecular-orbital-diagram-of-CoF-6-3-complex-with-six-p-donor-ligands.png)















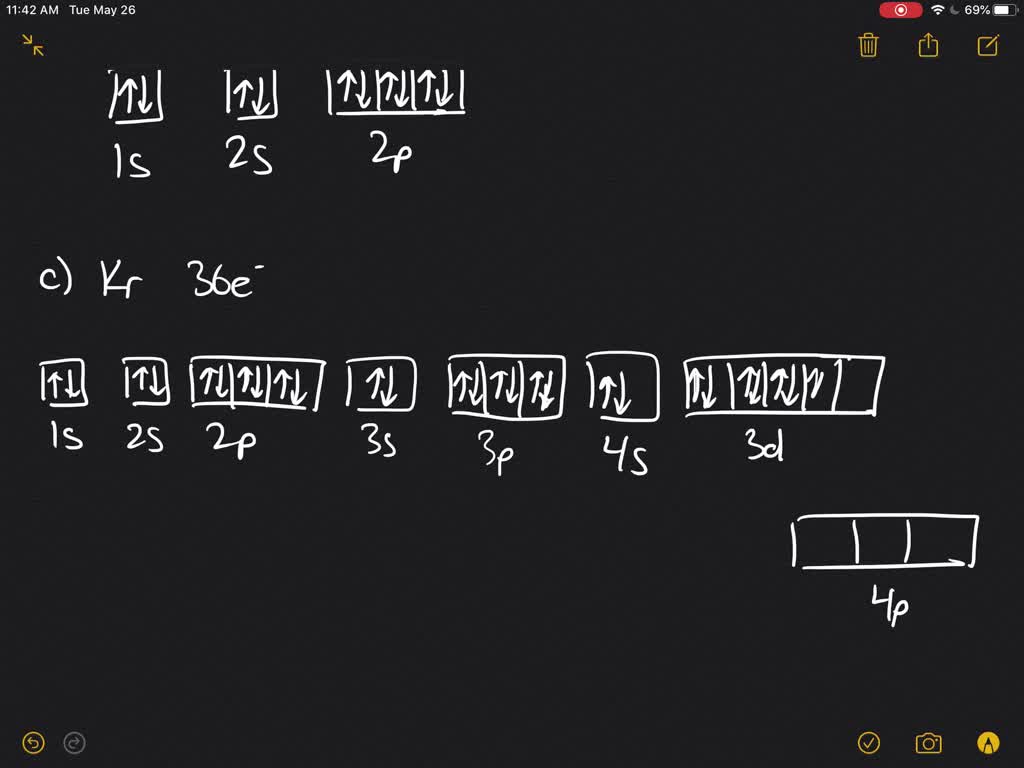









0 Response to "42 orbital diagram for as"
Post a Comment