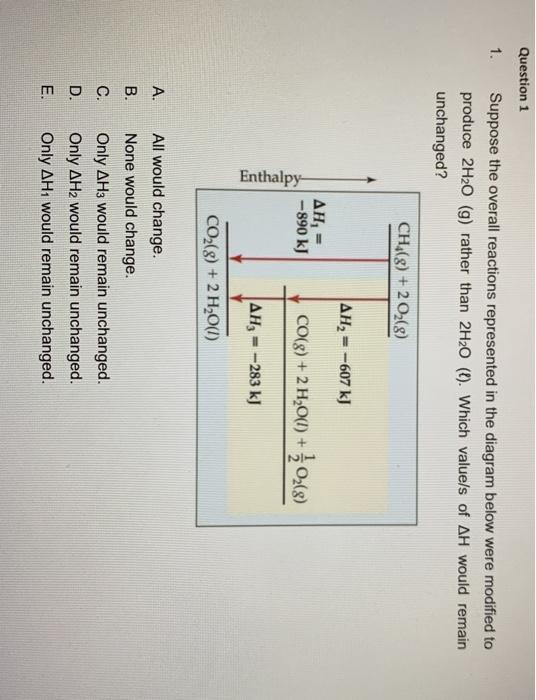
40 which of the following enthalpies of reaction would the reaction represented by the diagram have
Thermochemical Changes - ppt download 32 Enthalpy Change Enthalpy change can be communicated in the following four ways The molar enthalpy of a process, such as formation or combustion 46 Molar enthalpy of a reaction Note: if the balanced chemical reaction indicates that the specified compound has a coefficient other than 1 the... Chemistry 4.09 Quiz: Writing Thermochemical Equations Flashcards ... Which of the following enthalpies of reaction would the reaction represented by the diagram have? ΔH < 0 ...
5.7: Enthalpy Calculations - Chemistry LibreTexts Calculating enthalpies of reaction from heats of formation or combustion data, and applying it to real systems. If an equation has a chemical on the opposite side, write it backwards and change the sign of the reaction enthalpy. If the equation has a different stoichiometric coefficient than the one you...
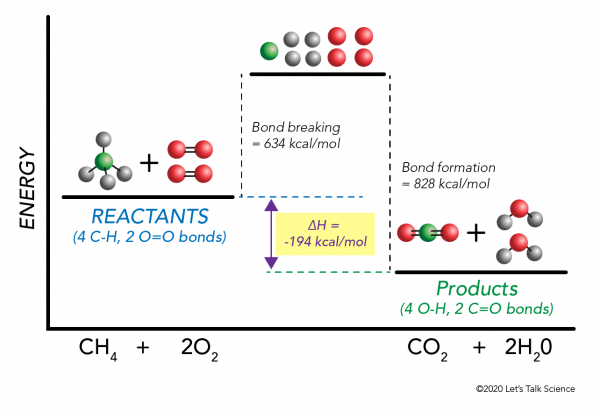
Which of the following enthalpies of reaction would the reaction represented by the diagram have
Chemistry 4.09: Writing Thermochemical Equations Flashcards ... Which of the following enthalpies of reaction would the reaction represented by the diagram have? ΔH < 0 ... sem 2 chem 4.09 Flashcards | Quizlet Which of the following enthalpies of reaction would the reaction represented by the diagram have? ... How to Calculate Enthalpies of Reaction | Sciencing In a combustion reaction, chemical energy is transformed into thermal energy. In reactions that change molecular makeup, energy is either required or Example 2: Calculate the enthalpy change per mole of carbon monoxide for the reaction of carbon monoxide with oxygen to give carbon dioxide.
Which of the following enthalpies of reaction would the reaction represented by the diagram have. 6 g What is the standard enthalphy change ΔH Which of the following expressions is equivalent to ΔHo for the reaction represented above? A x + y B x - y C x + 2y D 5. How much heat is released or absorbed when 0.050 mol of Cl2(g) is formed from KCl(s)? 87.4 kJ is released 43.7 kJ is released 43.7 kJ is absorbed 87.4 kJ is absorbed 6. Enthalpies for Different Types of Reactions: Enthalpy, Videos... We can calculate the reaction enthalpy by subtracting the sum of enthalpies of all the reactants from that of the products. We can express it as , e.g. It is important to remember that we should balance the thermochemical equation in such a way that it represents the formation of a single mole of the... PDF Types of chemical reactions Redox reaction Reduction Single displacement reaction. Solubility rules State symbols Symbol equation Synthesis reaction Chemical equations. In any chemical reaction, the atoms of the different elements combine together to form a new substance called a compound. Enthalpies of Reaction | PDF | Enthalpy | Chemical Reactions Enthalpies of Reactions Skills to develop Calculate the enthalpies of reactions from bond energies. Calculate Enthalpy of Reaction from Enthalpy of Formation A similar cycle can be devised to calculate The enthalpies of formation are negative, and we have the following diagram.
PDF Chemistry 30 2017 Released Diploma Examination Items II The reaction absorbs energy from the surroundings. III The reaction stores energy in chemical bonds. 8. A correct statement about the reaction represented by the enthalpy diagram above is that 9. For the forward reaction, which of the following rows describes the reaction type and the... A Thousand Questions With Paimon (Paimon Quiz) - All... - GameWith Q. Which Of The Following Statements About Elemental Reactions Is False? Anemo Can Have A Swirl Reaction With Geo. Q. When Fighting Electro Hypostasis: Aleph, Which Of The Following Elements Is Unable To Decrease The Hp Of Its Prisms? 3 Ways to Calculate the Enthalpy of a Chemical Reaction - wikiHow Any chemical reaction involves two categories of chemicals — products and reactants. Products are the chemicals created by the reaction, while reactants Enthalpies of formation are set ∆H values that represent the enthalpy changes from reactions used to create given chemicals. If you know the... 4.09 Writing Thermochemical Equations Quiz - Quizlet Which enthalpy of reaction would the reaction represented by the diagram have? Diagram: - ...
exercises - thermochemistry - chemistry the central science Why is this enthalpy change called the enthalpy of formation of the involved product? 5.34 (a) Under what condition will the enthalpy change of a process equal the amount of heat transferred Would the measured heat change represent ΔH or ΔE? If there is a difference, which quantity is... which of the following enthalpies of reaction would the ... In chemical reactions, the internal energy represents the total energy of the system and is often called Enthalpy. The x-axis represents the reaction progress. Chemical reactions proceed (or are read) from left to right. Therefore, looking at the potential energy diagram, the reactants are usually... PDF enthalpy.rtf 1. Enthalpy changes of reaction can be determined indirectly from average bond enthalpies and (b) The figure below is an incomplete enthalpy profile diagram for the reaction between methane and (i) Use these data to calculate the enthalpy change for the reaction of hydrazine with oxygen, as... Ellingham Diagrams (all content) | Partial pressure of reacting gas We shall consider the following reactions on the Ellingham diagram below: the oxidation of silver to form Ag2O (s); and the oxidation of cobalt to We can hence see that the relative stability of the oxide of cobalt compared with the oxide of silver is due to the much larger standard enthalpy of reaction.
Project Planning: Putting It All Together Week 2 Quiz Answer Which project plan component does this represent? Which of the following strategies follow project plan best practices? Select all that apply. Which of the following are relevant project documents you should link to in your project plan?
Transition metal The following scheme shows some reactions of chromium compounds in aqueous solution. (a) Identify the It reacts with aqueous iron(II) ions as shown in the equation below. The enthalpy change for this These are not shown in the diagram. (a) Name the type of bond that is represented by the...
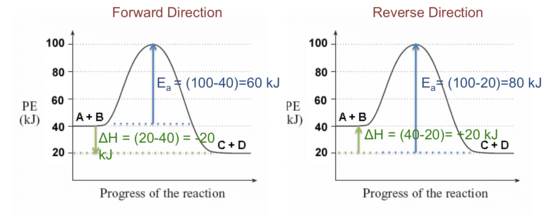
36 what is the enthalpy of reaction from the following energy ... Feb 08, 2022 · From the given energy diagram we conclude that this is an exothermic reaction diagram. Thus, the enthalpy of reaction is, -20 kJ The enthalpy of reaction is the amount of heat that is required or to be released in order to convert the reactants to the products. this can be calculated through the equation, δh = h (products) - h (reactants)
ChemTeam: Hess' Law - using standard enthalpies of formation The enthalpy of a given chemical reaction is constant, regardless of the reaction happening in one step or many steps. I'll explain the above equation using an example problem. Example #1: Calculate the standard enthalpy of combustion for the following reaction
PDF Measuring the Enthalpy Change for a Reaction Experimentally Calculating the enthalpy change of reaction, DHr from experimental data. General method 1. Using q= m x cp x DT calculate energy change for quantities Finally add in the sign to represent the energy change: if temp increases the reaction is exothermic and is given a minus sign eg -1470 kJ mol-1.
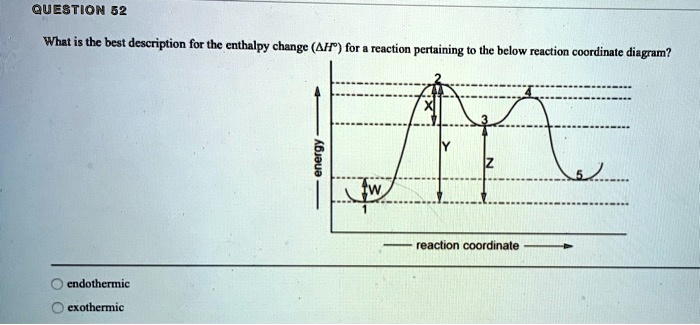
Select the correct answer. What is the enthalpy ... - Brainly.com Jun 12, 2020 · Select the correct answer. What is the enthalpy for the reaction represented in the following energy diagram? A. +200 kJ B. +350 kJ C. +150 kJ D. +250 kJ - 16811222
Enthalpy of Neutralisation Chemistry Tutorial (ii) enthalpy change for the reaction = -heat produced. A schematic diagram of a simple polystyrene foam cup calorimeter is shown below: A known amount of a reactant, such as a dilute solution of a base, is placed in the polystyrene cup (insulated vessel in the diagram).
Genshin Impact A Thousand Questions With Paimon Quiz Event... Q: What reaction will occur when a Pyro Elemental Attack is used against Anemo Hypostasis: Beth? A: Swirl. Q: At which of the following Liyue restaurants can you Q: Which of the following Ley Line Disorders has not featured in a domain? A: Characters being periodically inflicted with High Voltage.
Hess's Law and enthalpy change calculations Most calculations follow from it. However many stages the reaction is done in, ultimately the overall enthalpy change will be the same, because the positions of the reactants and products on an enthalpy diagram will always be the same.
Standard Enthalpy of Formation and Reaction | Boundless Chemistry The standard enthalpy of reaction is the enthalpy change that occurs in a system when a chemical Enthalpy change for a reaction is independent of the number of ways a product can be obtained, if Graphical representation of Hess's law: The net reaction here is A being converted into D, and the...
How is the enthalpy of reaction related to the enthalpies of formation... In an exothermic reaction heat is released, so the enthalpy of the products would be lower than the enthalpy of the reactants Which of the following is true of a reaction with a negative enthalpy? C: The enthaphy of the What part of a potential energy diagram reveals the enthalpy of reaction?
Enthalpy Change of Reaction & Formation - Thermochemistry... This chemistry video tutorial focuses on the calculation of the enthalpy of a reaction using standard molar heats of formation, hess law, and calorimetry.
Chemistry chapter 6 smartbook Flashcards | Quizlet In the enthalpy diagram shown, the reactants are _____ in energy than the ... Which of the following reactions would have an enthalpy change equal to ΔHfo?
Standard enthalpy of reaction - Wikipedia The standard enthalpy of reaction (denoted. or. ) for a chemical reaction is the difference between total reactant and total product molar enthalpies, calculated for substances in their standard states. This can in turn be used to predict the total chemical bond energy liberated or bound during reaction...
5.3 Enthalpy - Chemistry 2e | OpenStax | Combustion Reaction The enthalpy change of a reaction depends on the physical states of the reactants and products Because the equation, as written, represents the reaction of 8 mol KClO3, the enthalpy change is. Enthalpies of combustion for many substances have been measured; a few of these are listed in...
Determination of the Enthalpy Change of a Reaction | 123 Help Me This means that therefore the enthalpy change of a reaction can be measured by the calculation of 2 other reactions which relate directly to the reactants used in the first reaction and provided the same reaction In the reaction above, the overall order of reaction is given by the following: order=m+n...
Genshin Impact: Paimon 'A Thousand Questions' Quiz Answers The Burning reaction itself deals DMG. Which of the following monsters will not emerge from a Blossom of Revelation challenge? Which of the following statements about Keqing is false? Keqing can perform her Elemental Skill, Stellar Restoration, while in mid-air.
Chemistry 4.09 Quiz: Writing Thermochemical Equations ... In which of the following thermochemical equations would the ΔH be considered a heat of solution? NH4Cl (s) → NH4 (aq) + Cl- (aq), ΔH = +14.8 kJ/mol Which of the following enthalpies of reaction would the reaction represented by the diagram have?
What are some different ways to calculate the enthalpy of... - Quora Chemical reaction is the interaction of two or more elements or compound at the sub atomic level. Results of chemical changes includes the following For the sake of simplicity, the subscript "sys" will be left off the symbol for both the internal energy of the system and the enthalpy of the system...
Thermochemistry Flashcards | Quizlet Use the following reaction enthalpies to find the enthalpy of the first reaction. ... In the heat of formation equation for CH3CH2OH, what would be the ...
Heat of Reaction | Measure Reaction Enthalpy The heat of reaction, or reaction enthalpy, is an essential parameter to safely and successfully scale-up chemical processes. The heat of reaction is the energy that is released or absorbed when chemicals are transformed in a chemical reaction. It describes the change of the energy content...
How to Calculate Enthalpies of Reaction | Sciencing In a combustion reaction, chemical energy is transformed into thermal energy. In reactions that change molecular makeup, energy is either required or Example 2: Calculate the enthalpy change per mole of carbon monoxide for the reaction of carbon monoxide with oxygen to give carbon dioxide.
sem 2 chem 4.09 Flashcards | Quizlet Which of the following enthalpies of reaction would the reaction represented by the diagram have? ...
Chemistry 4.09: Writing Thermochemical Equations Flashcards ... Which of the following enthalpies of reaction would the reaction represented by the diagram have? ΔH < 0 ...
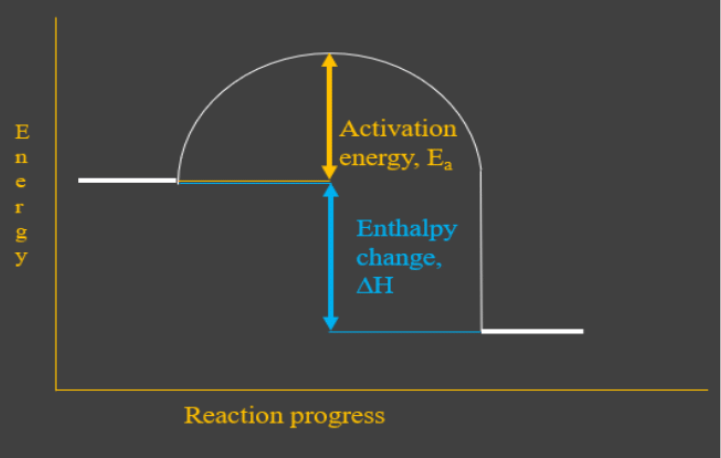
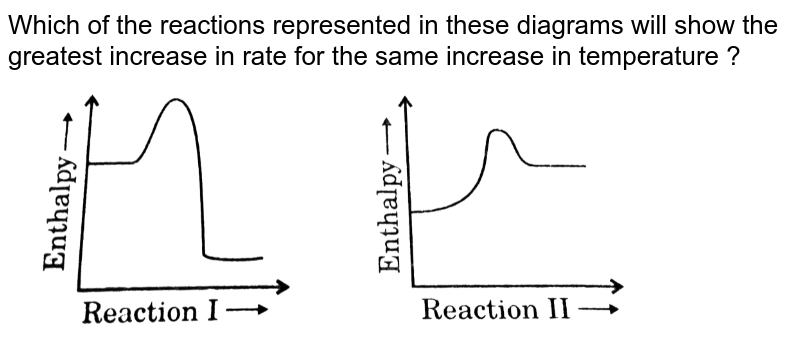



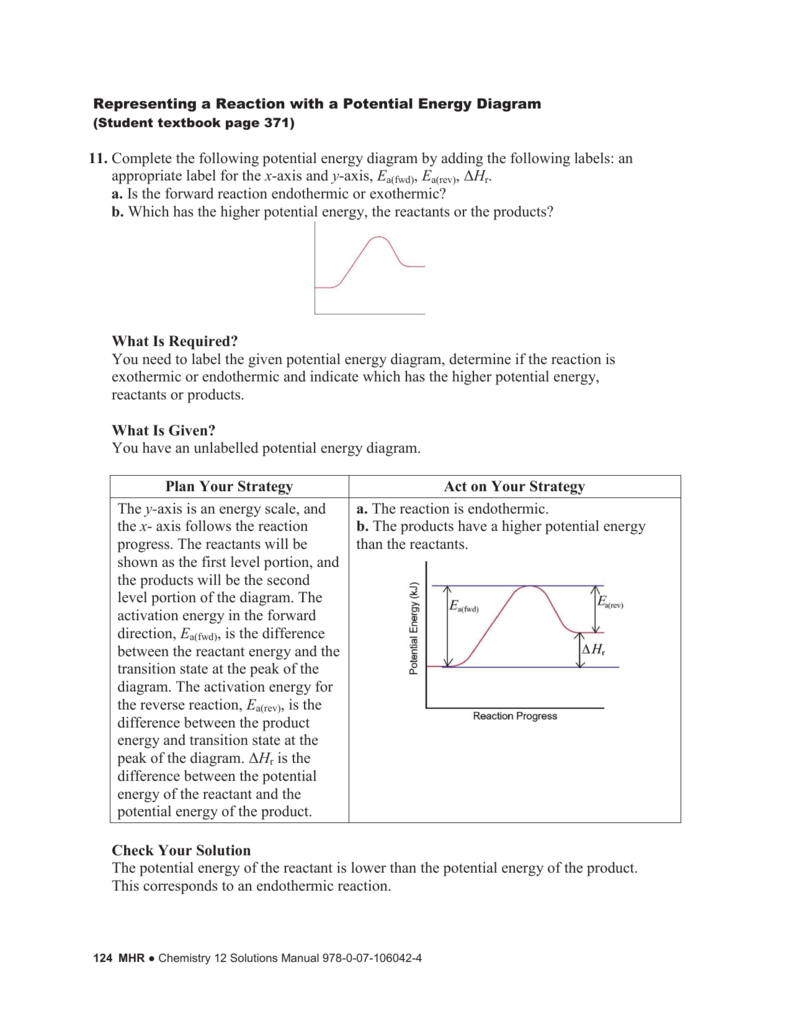

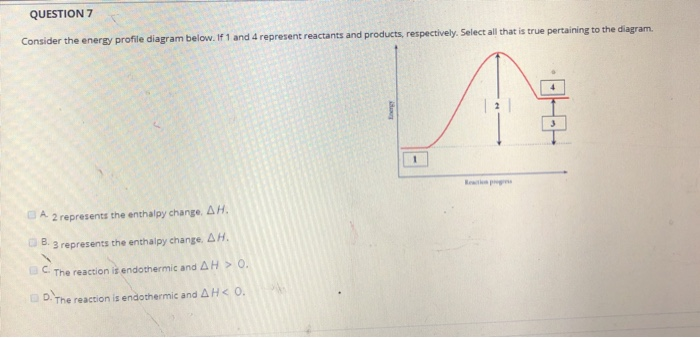




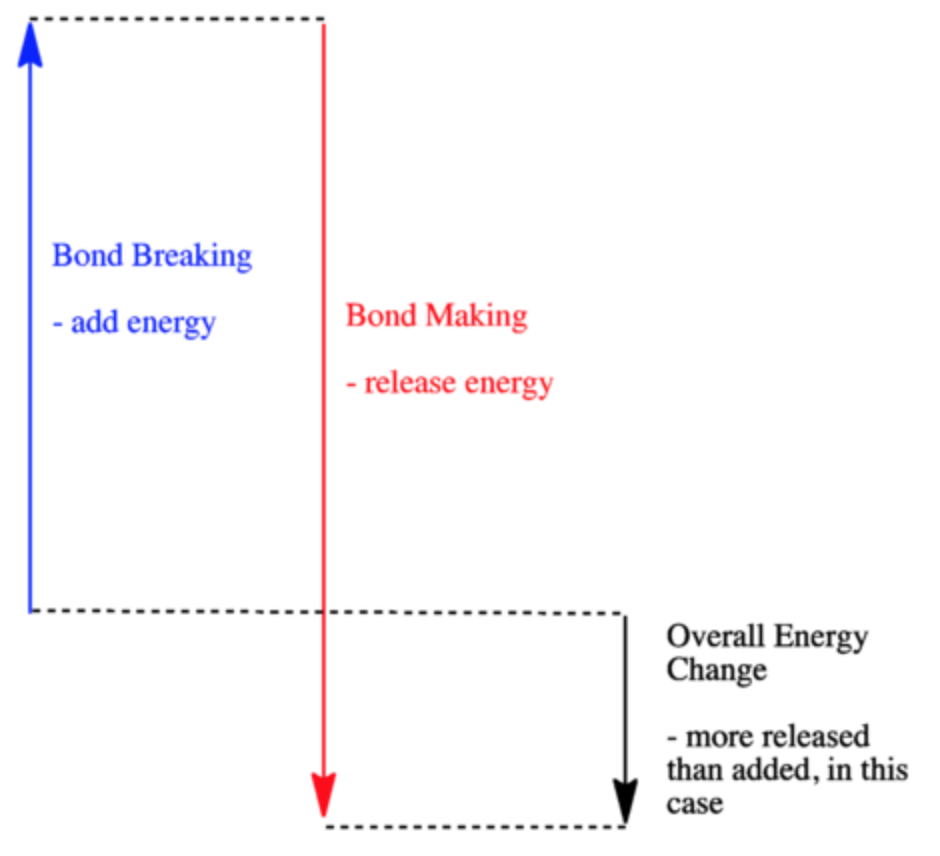
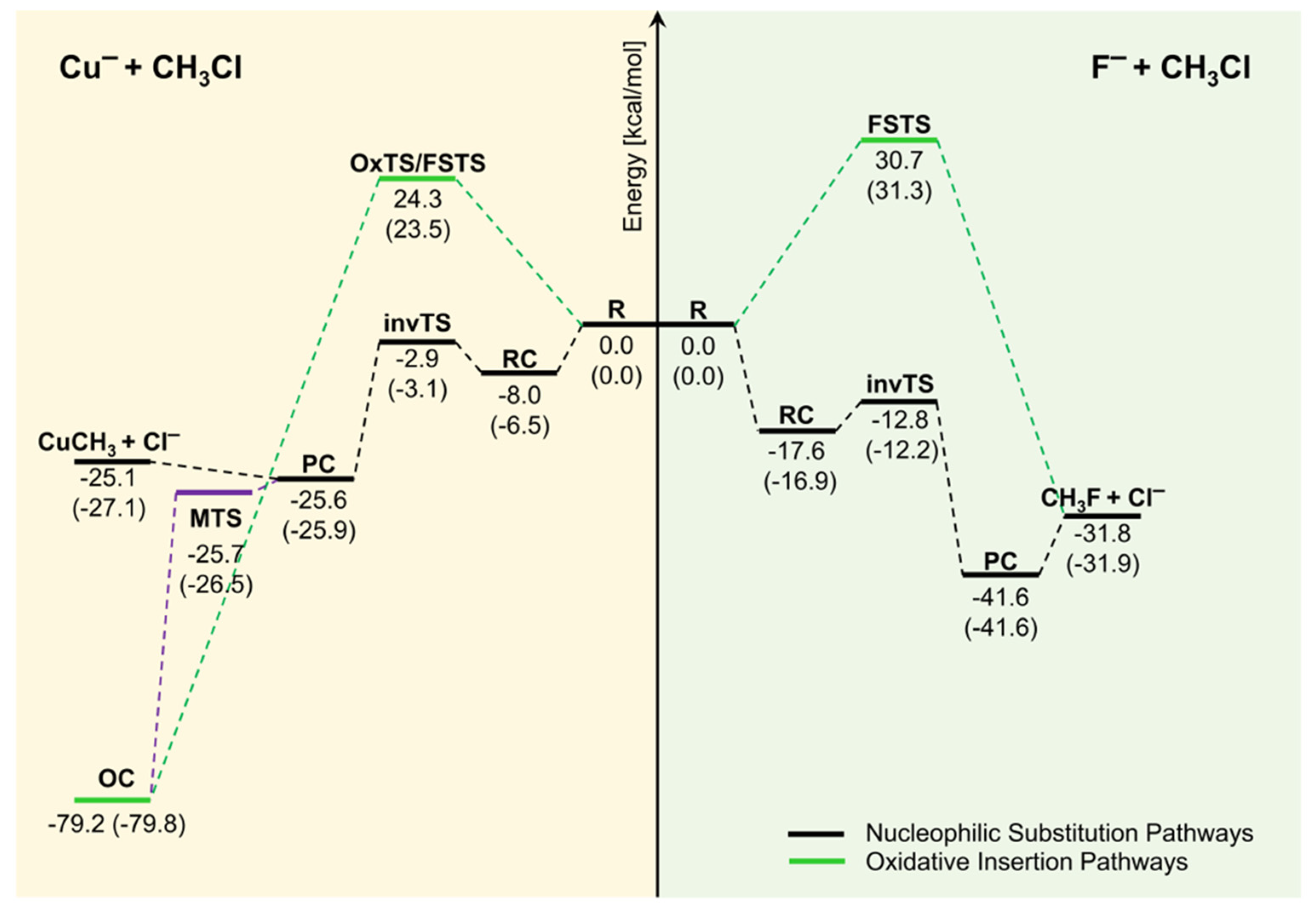



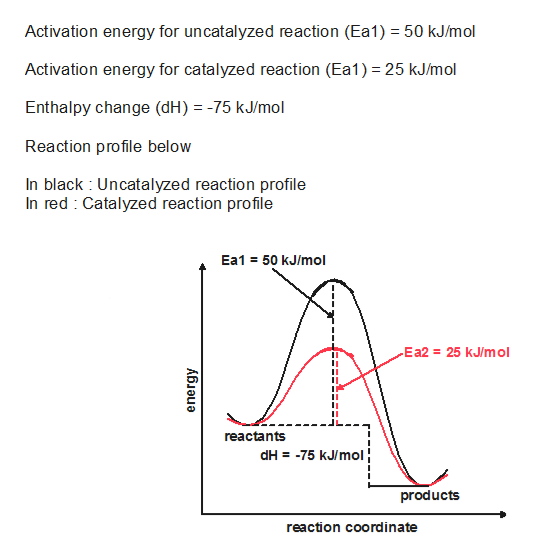




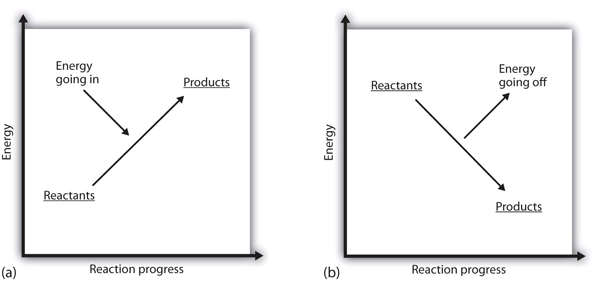


0 Response to "40 which of the following enthalpies of reaction would the reaction represented by the diagram have"
Post a Comment