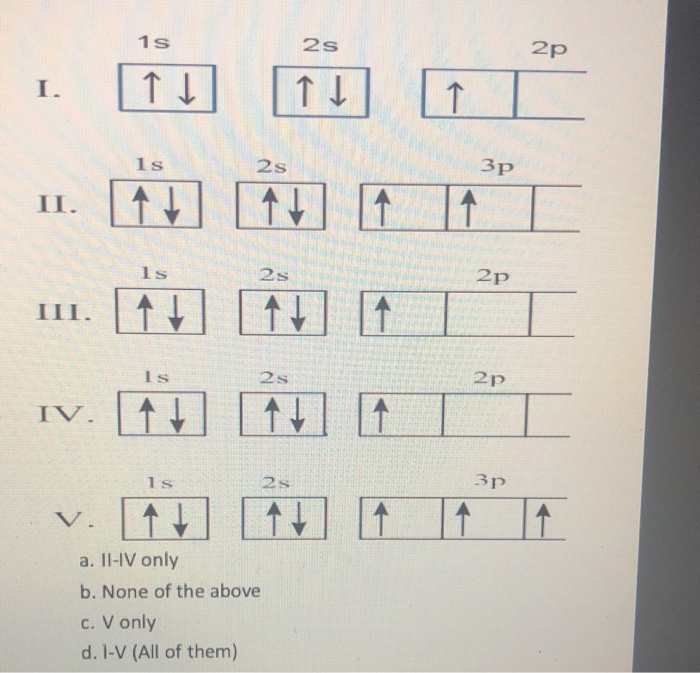
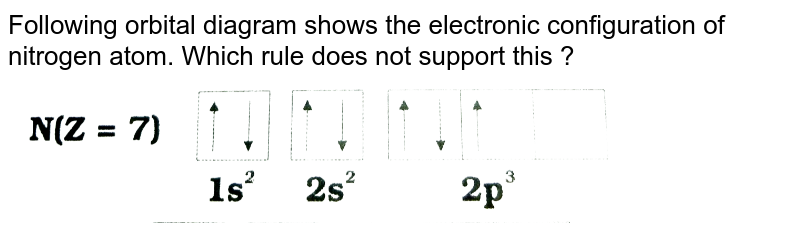
39 orbital diagram for nitrogen
Solved Fill in the atomic orbital diagram for nitrogen ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (17 ratings) Transcribed image text: Fill in the atomic orbital diagram for nitrogen. Answer Bank Energy Construct the orbital diagram for nickel. 1000 Answer Bank Energy 2 _ _ _. Orbital Diagram For Nitrogen (N) | Nitrogen Electron ... Feb 15, 2021 · If you are still not getting the Nitrogen Electron Configuration of the element nitrogen then, the full electronic configuration of nitrogen is written as the following; 1s 2 2s 2 2p 3. If we gave you brief information then, the first two electrons lie in the 1s orbital, following the next 2 electrons, it comes under the 2s orbital.
Nitrogen Electron Configuration (N) with Orbital Diagram Jan 21, 2021 — When we write the electron configuration of N the first two electrons go in the 1s orbital. As 1s can only hold 2 electrons and the other next ...
Orbital diagram for nitrogen
Molecular Orbital Diagram of Nitrogen Molecule - Nature of ... Molecular Orbital Diagram of Nitrogen Molecule Video Lecture from Chapter Nature of Chemical Bond of Subject Chemistry Class 11 for HSC, IIT JEE, CBSE & NEET... Create the atomic orbital diagram for nitrogen. - Clutch Prep Create the atomic orbital diagram for nitrogen. Learn this topic by watching The Electron Configuration Concept Videos All Chemistry Practice Problems The Electron Configuration Practice Problems Q. Construct the orbital diagram of each atom or ion.TiTi2+Ti4+ Q. Write the corresponding electron configuration for the following pictorial ... Orbital filling diagrams - The Cavalcade o' Chemistry The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ...
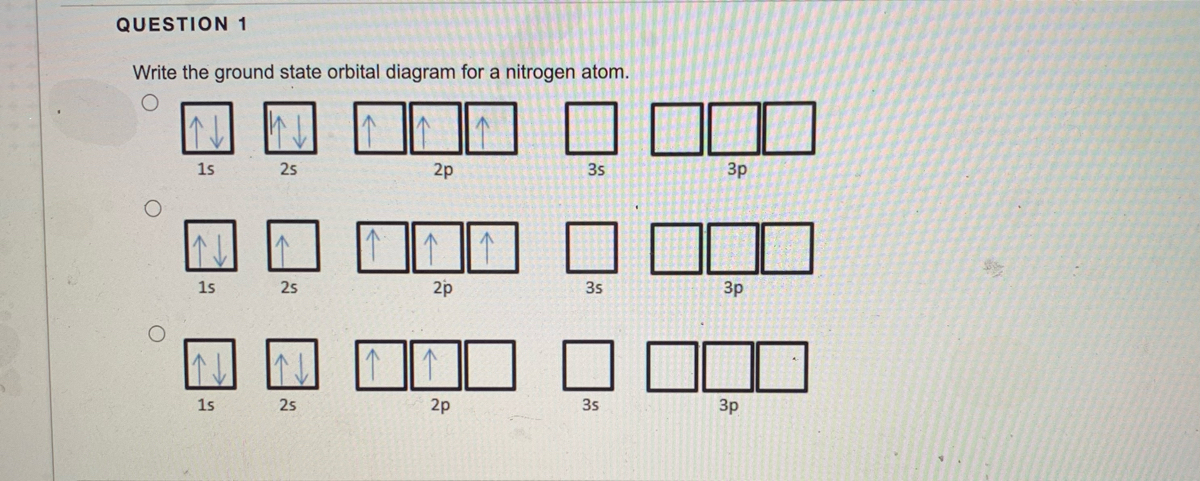
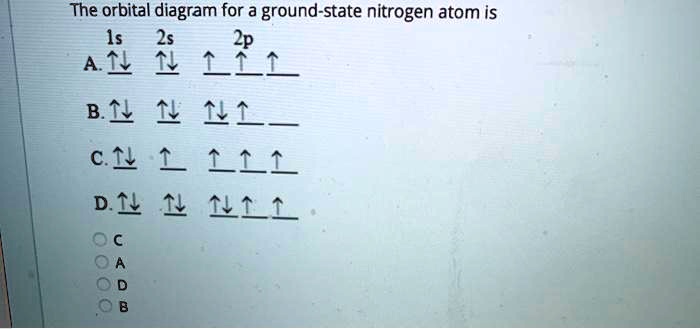
Orbital diagram for nitrogen. What is the atomic orbital diagram for nitrogen? What is the atomic orbital diagram for nitrogen? The remaining three electrons will go in the 2p orbital. Therefore the N electron configuration will be 1s22s22p3. The configuration notation for Nitrogen (N) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the Nitrogen atom. How to Do Orbital Diagrams - Sciencing The first number is the principal quantum number (n) and the letter represents the value of l (angular momentum quantum number; 1 = s, 2 = p, 3 = d and 4 = f) for the orbital, and the superscript number tells you how many electrons are in that orbital. Orbital diagrams use the same basic format, but instead of numbers for the electrons, they ... 8 - Drawing Molecular Orbital Diagrams — Flux Science Molecular orbital diagram of diatomic nitrogen. Homonuclear molecular orbitals are formed between two elements that are the same, meaning that they are naturally symmetrical and will perfectly overlap. However, before we fill out this diagram, Compare this MOD to the one above, particularly in the 2p region. Nitrogen(N) electron configuration and orbital diagram Orbital Diagram for Nitrogen (N) Nitrogen(N) excited state electron configuration. Atoms can jump from one orbital to another by excited state. This is called quantum jump. Ground state electron configuration of nitrogen is 1s 2 2s 2 2p 3. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z. Each sub-orbital can have a maximum of two electrons.
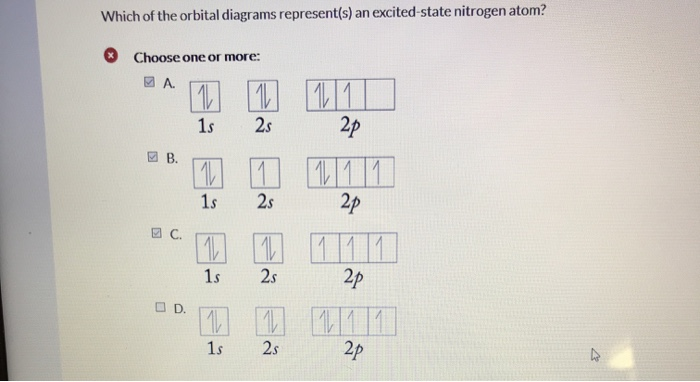
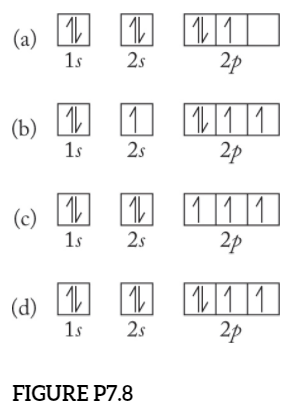
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Hydrogen (H) 2: Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11 ... Orbital Diagram For Nitrogen - Free PDF eBook Orbital Diagram For Nitrogen Free PDF eBooks. Posted on July 30, 2015. MO Diagrams for O2 and N2 - U of L Class Index. Drawing MO diagrams - always same number of MOs as AOs. ... N2 σ2s σ*2s σ* 2p π*2p π2p σ2p smaller ΔE. N2. Diamagnetic. Bond order of 3 (N≡N). Transition Metal Review.pdf. [ANSWERED] Which Of The Orbital Diagrams Represent(S) An ... Answer Electronic configuration of nitrogen in ground state is 1s2 2s2 2p3 or 1s2 2s2 2px1 2py1 2pz1. Hence, in excited state one of the 2s electron will jump to 2p orbital,so the excited state electronic configuration should be 1s2 2s1 2px2 2py1 2pz1. I BEG you Orbital Filling Diagram For Nitrogen - wiringall.com Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital. Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Nov 08 ...
NO2 Lewis Structure, Molecular Geometry, Hybridization ... Lewis Structure of NO2. A molecule of nitrogen dioxide consists of one nitrogen atom and two atoms of oxygen. Let us look at the periodic table. Nitrogen belongs to group 15 ( or group 5) and has an atomic number of 7, therefore has a valency of 5. Oxygen belongs to group 16 ( or group 6) and has an atomic number of 8, therefore a valency of 6. Nitrogen Bohr Model - How to draw Bohr diagram for ... Summary. The Bohr model of Nitrogen is drawn with only two electron shells, the first shell contains 2 electrons and the second shell contains 5 electrons. Nitrogen is neutral and its atomic number is 7, hence, the number of protons and electrons available for its Bohr diagram is also 7. The number of neutrons for the Bohr diagram of Nitrogen ... Orbital Filling Diagram For Nitrogen - schematron.org Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1. The 2p . Orbital filling diagrams essentially just turn this big list of electron locations . In the same way, the orbital filling diagram for nitrogen will be.Given the same amount of absorbed solar energy coming in, the amount of IR escaping to space at the top of the ... Molecular orbitals in Nitrogen - ChemTube3D Orbital-orbital Interactions and Symmetry Adapted Linear Combinations; Metal reaction mechanisms. Point groups of polyhedral structures; Distortions of a regular Octahedron; Distortions of a octahedral complex with chelating ligands; Berry pseudorotation; Octahedral [Ru(en) 3] 2+ enantiomers; Delta v lambda M(L-L) 3; Ligand Substitution Square ...
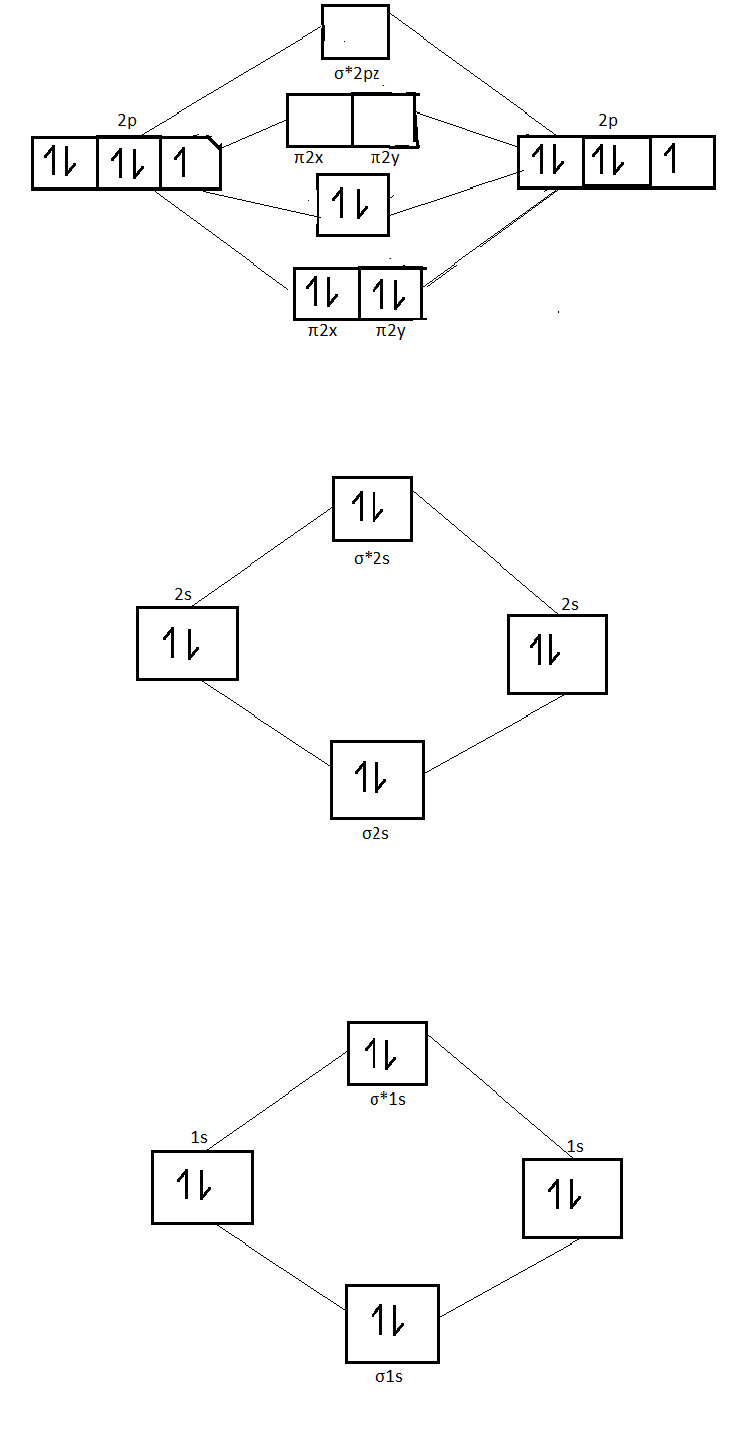
Molecular Orbital (MO) Diagram of N2 - YouTube Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or...
Manganese(Mn) electron configuration and orbital diagram In the manganese (Mn) ground-state electron configuration, the five electrons of the 3d orbital are located in the d xy, d yz, d zx, d x2-y2 and d z2 sub-orbitals. The d-orbital has five sub-orbitals. The sub-orbitals are d xy, d yz, d zx, d x2-y2 and d z2. Each sub-orbital can have a maximum of two electrons.
Electron Configuration for Nitrogen (N) - UMD Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital.
Nitrogen Orbital diagram, Electron configuration, and Valence ... The orbital diagram for nitrogen is drawn by following three principles – the Aufbau principle, Hund’s principle, Pauli’s exclusion principle. The nitrogen orbital diagram comprises three orbitals. The three orbitals are 1s, 2s, and 2p. The first two electrons will go in 1s orbital, the next two in 2s orbital, and the last three in 2p orbital.
Orbital Diagrams Flashcards - Quizlet Start studying Orbital Diagrams. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Nitrogen Orbital Filling Diagram - schemacheck.com Which is the orbital diagram for nitrogen? When we write the electron configuration of N the first two electrons go in the 1s orbital. As 1s can only hold 2 electrons and the other next two electrons for Nitrogen (N) go in the 2s orbital. The three electrons that are remained will go in . Bromine Orbital Diagram.
What is the orbital diagram of nitrogen? Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital.
N2+ Mo Diagram - schematron.org The correlation diagrams for nitrogen and carbon monoxide and the first are nearly parallel to the corresponding orbital energy curves. Bond order for N2 is 3; bond order for N2- is and bond order for N2+ is I have not included pictures of the MO diagrams that show the orbital energies. N2+ has less bond energy.
Draw the molecular orbital diagram of N2N2 + N2 Write ... Electrons of nitrogen are to be filled in this diagram. Left side represents the configuration of one atom of nitrogen molecule and the right side represents the second atom of nitrogen molecule. Atomic number of nitrogen is seven. Therefore in ${N_2}$ there are a total fourteen electrons. Molecular orbital diagram of ${N_2}$ is shown below:
Orbital filling diagrams - The Cavalcade o' Chemistry The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ...
Create the atomic orbital diagram for nitrogen. - Clutch Prep Create the atomic orbital diagram for nitrogen. Learn this topic by watching The Electron Configuration Concept Videos All Chemistry Practice Problems The Electron Configuration Practice Problems Q. Construct the orbital diagram of each atom or ion.TiTi2+Ti4+ Q. Write the corresponding electron configuration for the following pictorial ...
Molecular Orbital Diagram of Nitrogen Molecule - Nature of ... Molecular Orbital Diagram of Nitrogen Molecule Video Lecture from Chapter Nature of Chemical Bond of Subject Chemistry Class 11 for HSC, IIT JEE, CBSE & NEET...









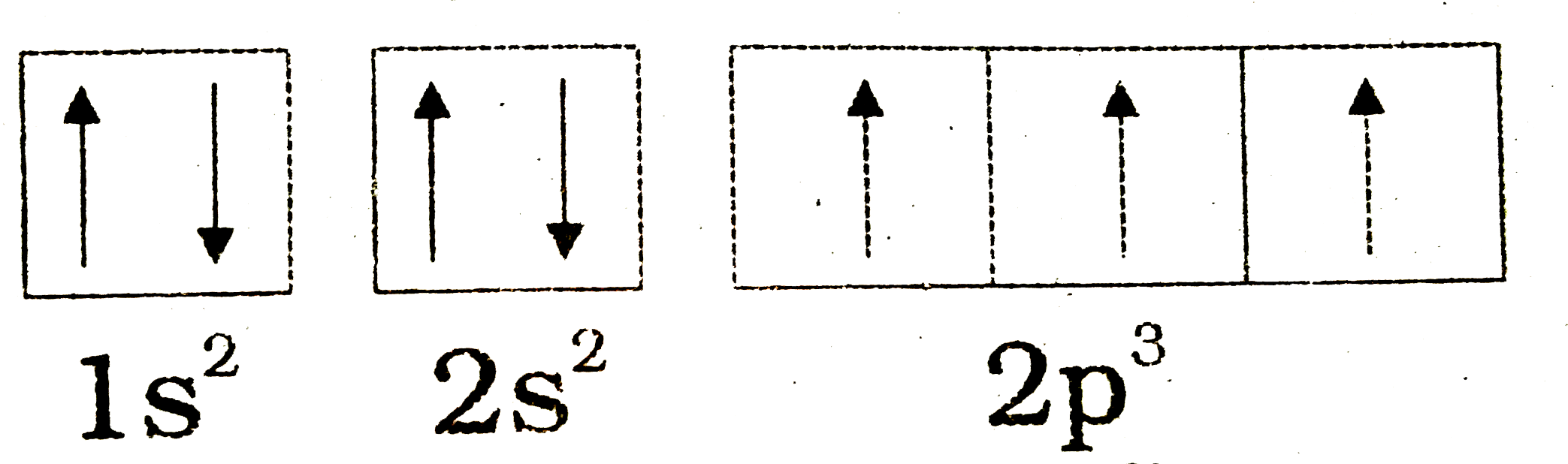














0 Response to "39 orbital diagram for nitrogen"
Post a Comment