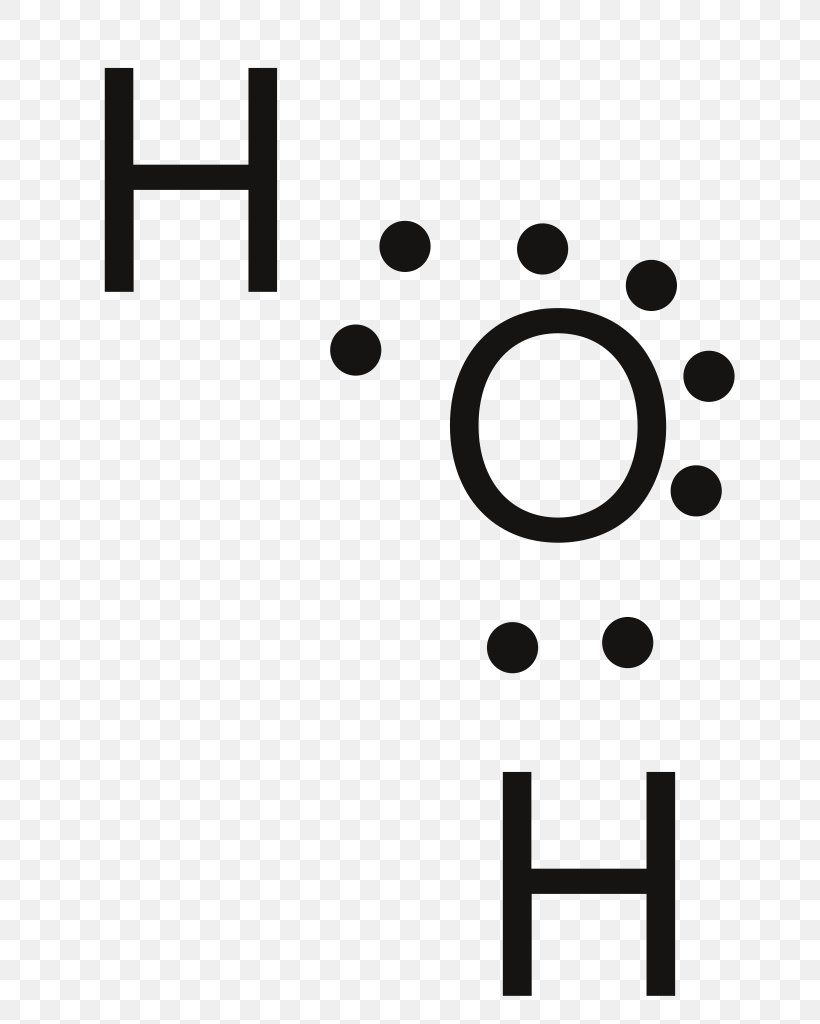
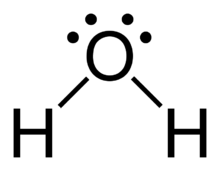
39 lewis diagram for h2o
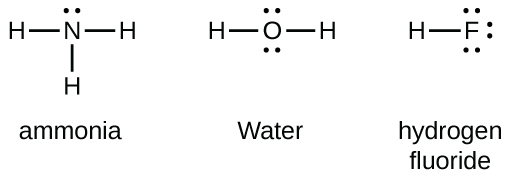
In the Lewis Structure of H2O2, there are three single bonds formed having two H-O bonds and one O-O bond. There are two lone pairs of electrons on each Oxygen atom; thus, there are four lone pairs of electrons for H2O2. As each Oxygen atom forms an sp3 hybrid orbital, H2O2 has sp3 hybridization. Part 1—Lewis Structures: Hand out the worksheets. Explain that students are to sketch out a Lewis dot structure for each of the 12 molecules listed on the worksheet: CH 4, CO 2, NH 3, H 2 O, N 2, SO 2, O 2, O 3, CO, CO 3, NO 3, and CF 2 Cl 2 (CFC, chlorofluorocarbon). Instructions are provided on the worksheet.
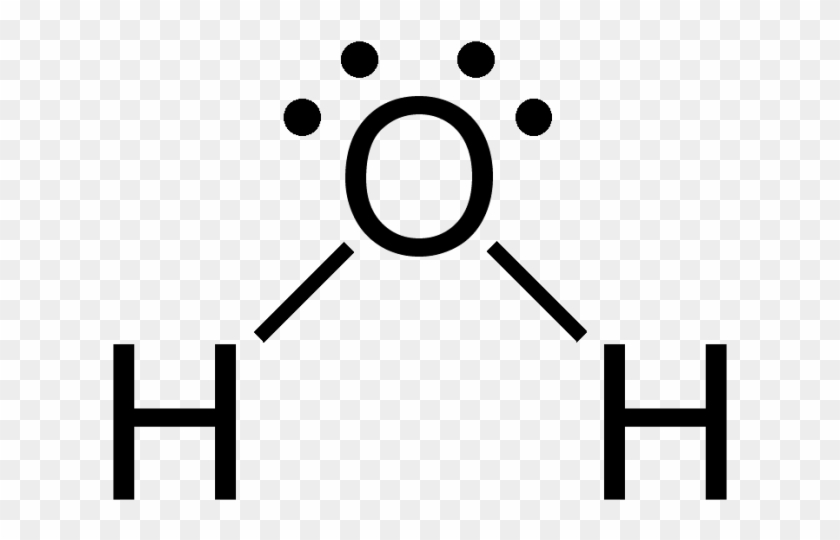
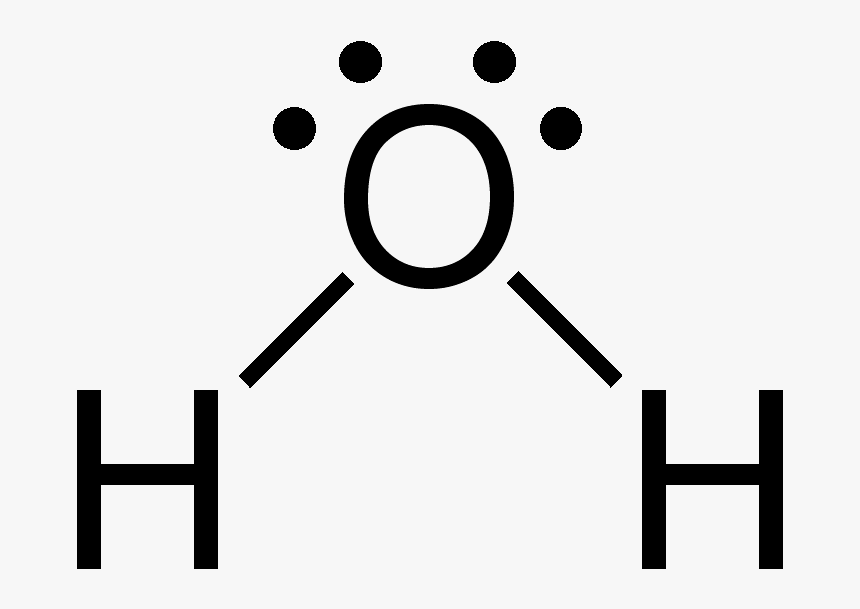
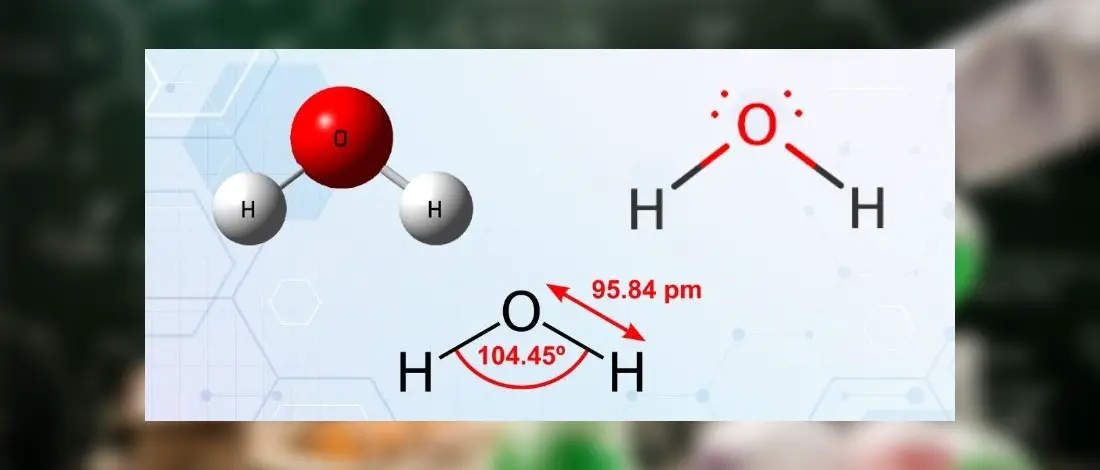
We need to know how the Lewis structure is drawn for the H2O molecule here: It is eight to make a single H2O molecule. From this, it can be seen that the central atom of a single hydrogen-oxygen-hydrogen atom is bent; Draw the lewis diagram: From this, it can be shown that the atomic geometry of a single H2O molecule is changed.
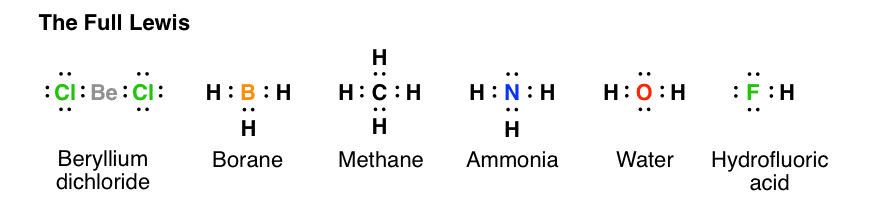
Lewis diagram for h2o
SO2 Lewis structure H 2 O Lewis structure Step 01: calculation of total valence electrons of H2O Step 02: Central atom =O. Step 03: Connect three atoms via single bonds. Step 04: Put remaining valence electrons as lone pairs. Step 05: Turn lone pairs into bonds as each atom fulfill electron octet rule. H2O Lewis structure NH 3 Lewis structure ... you can find a procedure for drawing lewis structures at this location.for h₂o, o must be the central atomthe skeleton structure is h-o-h.o has 6 valence electrons, and each h has one.you must arrange 8 electrons in pairs so that o has 8 and each h has two electrons in its valence shell.the trial structure is you have eight valence electrons in … CO2 or carbon dioxide is considered as acid or can even be called Lewis Acid. The resonance structure accepts lone pairs of electrons, but the three lone pairs of electrons are in the oxygen molecule. There are no lone pairs of electrons in CO 2. Although, when dissolved in water, CO 2 takes the form of carbonic acid using this formula.
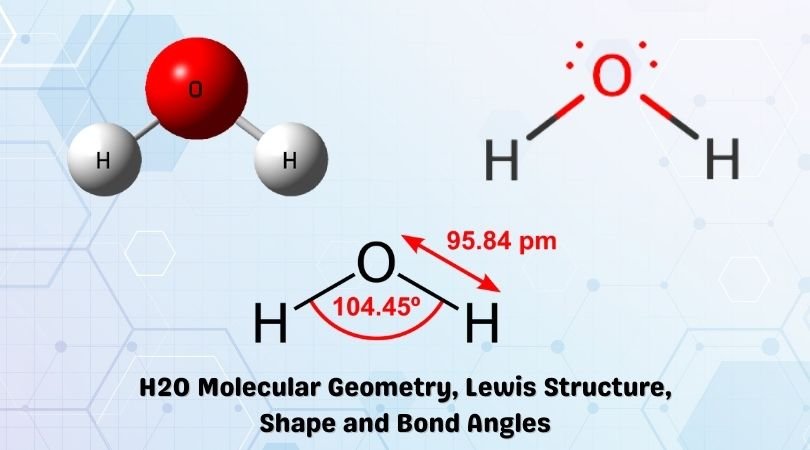
Lewis diagram for h2o. What is the molecular geometry of H2O? Water has 4 regions of electron density around the central oxygen atom (2 bonds and 2 lone pairs). These are arranged in a tetrahedral shape. The resulting molecular shape is bent with an H-O-H angle of 104.5°. What is the molecular geometry of the water molecule? H2o lewis structure 3d model . 022 lewis diagrams and vsepr modelsin this video paul andersen explains how you can use lewis diagrams and vsepr models to make predictions about molecules. Formaldehyde methanal h2co is a trigonal planar molecule ax3 geometry 120 degree bond angle. Drawing the lewis structure for h 2 o. H2o lewis structure 3d model Identify each violation to the octet rule by drawing a Lewis electron dot diagram. ClO; SF 6; Solution. a. With one Cl atom and one O atom, this molecule has 6 + 7 = 13 valence electrons, so it is an odd-electron molecule. A Lewis electron dot diagram for this molecule is as follows: First you draw Lewis structure of SO2. It help to understand about molecules. If you see carefully this structure. You can say that, It is not symmetric. it contains polar molecules. so you can say that it has not possibility to hydrogen bonding.
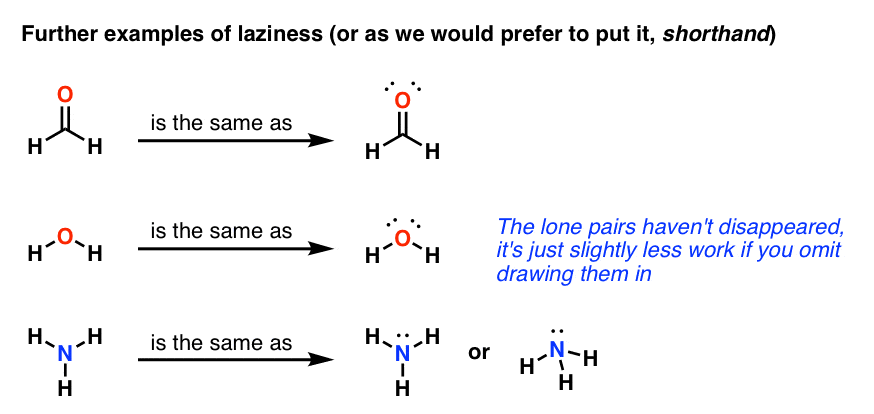
Another straight forward Lewis structure. You have a total of 8 valence electrons available to fill the octets of Oxygen and Hydrogen. Remember that Hydrogen ...Oct 24, 2016 · Uploaded by Wayne Breslyn 5.3: Lewis Diagrams. Lewis used simple diagrams (now called Lewis diagrams) to keep track of how many electrons were present in the outermost, or valence, shell of a given atom. The kernel of the atom, i.e., the nucleus together with the inner electrons, is represented by the chemical symbol, and only the valence electrons are drawn as dots ... What is the correct Lewis structure for H2O? The skeleton structure is H-O-H. O has 6 valence electrons, and each H has one. You must arrange 8 electrons in pairs so that O has 8 and each H has two electrons in its valence shell. You have eight valence electrons in your trial structure, so it has the correct number of electrons. This is the Lewis structure of the H2O molecule that has two single bonds between Oxygen and Hydrogen. As a result, there are two lone pairs in this molecule and two bonding pairs of electrons. H2O Hybridization When two atoms share electrons and form bonds, there is the formation of hybridized orbitals.
Oxygen has 6 valence electrons in its outer shell, but as we know there are 4 oxygen atoms in this molecule. Hence we will multiply the number by 4. So now there are 24 valence electrons for all oxygen atoms. However hydrogen has two valence electrons in this structure. = 6+24+2 = 32 valence electrons Oct 28, 2015 — It should look like this with the single line representing the sharing of two electrons between them.1 answer · https://simple.wikipedia.org/wiki/Lewis_structure Explanation: It should look like this with the single line representing the sharing of two electrons ... Two students made the Lewis dot diagrams of H2O. The diagrams are as shown.:01 H Н Н H Student A Student B Which student drew the correct Lewis dot diagram? Only Studenta Only Student B O Both Student A and Student B O Neither Student A nor Student B H2O is the molecular formula of water, one of the major constituents of the Earth. A single molecule is made up of two hydrogen atoms and one oxygen atom, ...Nov 27, 2020 · Uploaded by Wayne Breslyn
To review Lewis structures visit " Lewis Structures " Q21.5F Of the following complexes, name whether each pair is identical, geometric, or enantiomers. S21.5F Q21.6A [ Cr ( NH 3) 6] 3 + is yellow and [ Cu ( H 2 O) 4] 2 + is blue. Explain why. One of these is blue-green and the other is yellow. Indicate which and say why.
5 Step By Step Construction of SO2's Lewis Structure 1. Find The Total Valence Shells' Electrons The two molecules found on this compound belong to the VIA elements; therefore, they have six valence electrons, multiply it with the number of atoms found on each molecule and then add the products. Valence electrons from two oxygen atoms = 6 * 2 = 12
Draw a Lewis structure for H2O in which the central O atom obeys the octet rule, and answer the... Draw a Lewis structure for H2O in which the central O atom obeys the octet rule, and answer the following questions based on your drawing. The number of unshared pairs (lone pairs) on the central O atom is: The central O atom forms single bonds.
It is also known as an alkali metal oxide as it comprises two sodium and one Oxygen atoms. The compound is the base anhydride for NaOH, as when Na2O reacts with water, it produces NaOH. Na2O + H2O — 2NaOH The compound is widely used in ceramics and glasses.
Draw the Lewis structure of H2O. Include any nonbonding electron pairs. Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons. - CHONSPFBrClIXMore Request Answer Part B What is the electron geometry of H2O
CO2 or carbon dioxide is considered as acid or can even be called Lewis Acid. The resonance structure accepts lone pairs of electrons, but the three lone pairs of electrons are in the oxygen molecule. There are no lone pairs of electrons in CO 2. Although, when dissolved in water, CO 2 takes the form of carbonic acid using this formula.
you can find a procedure for drawing lewis structures at this location.for h₂o, o must be the central atomthe skeleton structure is h-o-h.o has 6 valence electrons, and each h has one.you must arrange 8 electrons in pairs so that o has 8 and each h has two electrons in its valence shell.the trial structure is you have eight valence electrons in …
SO2 Lewis structure H 2 O Lewis structure Step 01: calculation of total valence electrons of H2O Step 02: Central atom =O. Step 03: Connect three atoms via single bonds. Step 04: Put remaining valence electrons as lone pairs. Step 05: Turn lone pairs into bonds as each atom fulfill electron octet rule. H2O Lewis structure NH 3 Lewis structure ...
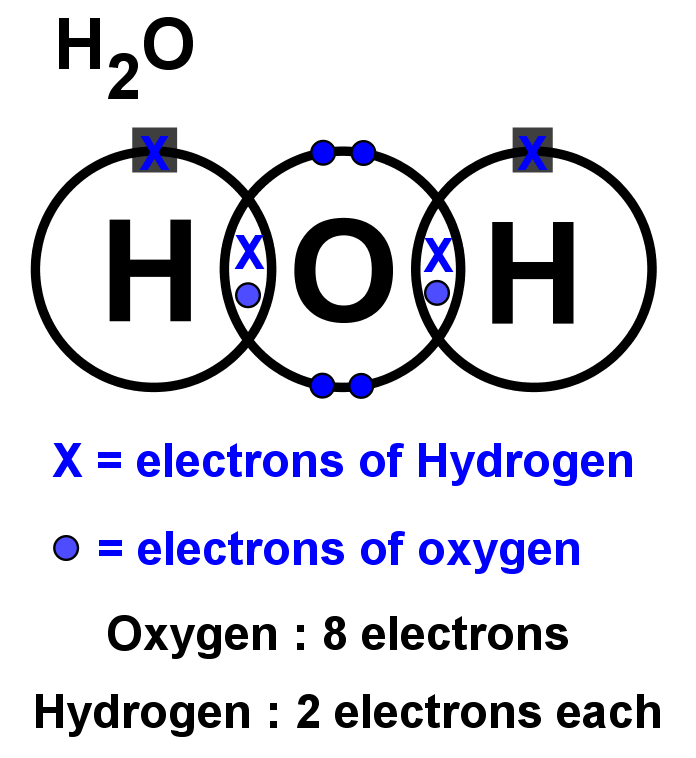
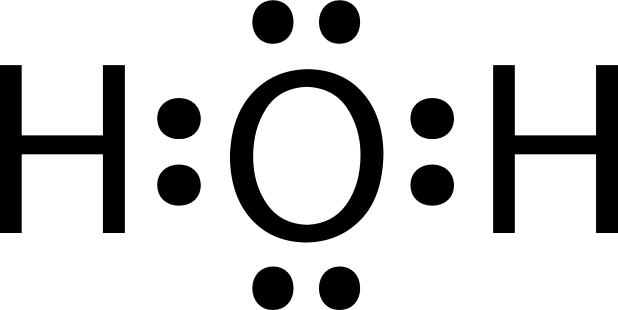





















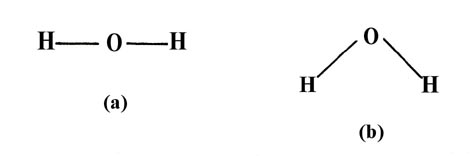


0 Response to "39 lewis diagram for h2o"
Post a Comment