38 Lewis Dot Diagram For Co
Carbon monoxide (CO) Molecule Lewis Structure CO lewis structure. In the lewis structure of carbon monoxide, both atoms have eight electrons in their valence shells. However, oxygen atoms has a +1 charge and carbon atom has a +1 charge. In next sections, we will draw CO lewis structure step by step. Steps of drawing lewis structure of CO molecule. There are guidelines (several steps) to ... Lewis Dot Structures Worksheet - Mr. Walsh's Class Draw the Lewis Dot Structure. Notes: Scientists use. Lewis Dot Structures. to show the valance electrons of an element as dots. Since bonding involves the valance shell electrons only, it is only necessary to illustrate those outer electrons.
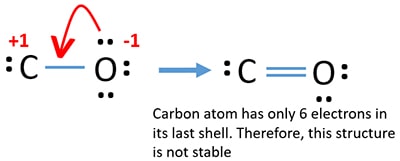
What is the Lewis structure of CO? | Socratic What is the Lewis structure of CO? Organic Chemistry Lewis Structures and Bonding Lewis Dot Diagram. 1 Answer Truong-Son N. Oct 30, 2015 This often looks wrong to a student who is used to seeing double bonds on oxygen. Students are typically taught an electron-counting method, which goes as follows: ...

Lewis dot diagram for co
History of molecular theory - Wikipedia In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.. The modern concept of molecules can be traced back towards pre-scientific and Greek philosophers such as Leucippus and Democritus who argued that all the universe is composed of atoms and voids. Lewis Dot Diagram Worksheet Answers - Worksheet Answer Key Lewis Dot Diagram Worksheet Answers - The principles of maths that a youngster researches are addition and reduction. It is difficult for a child to understand whatever at once. Educators and also parents can help him get the assistance he needs. Lewis Structure for CO - UMD The Lewis structure for CO has 10 valence electrons. For the CO Lewis structure you'll need a triple bond between the Carbon and Oxygen atoms in order to satisfy the octets of each atom while still using the 10 valence electrons available for the CO molecule. How to Draw the Lewis Dot Structure for Carbon monoxide It is helpful if you:
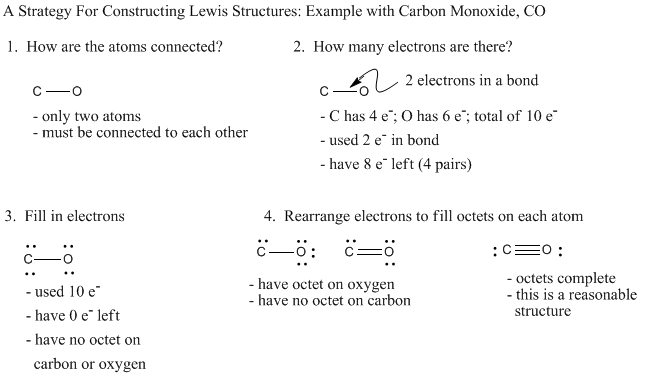
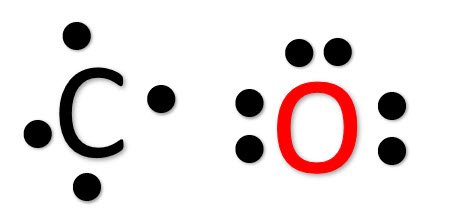
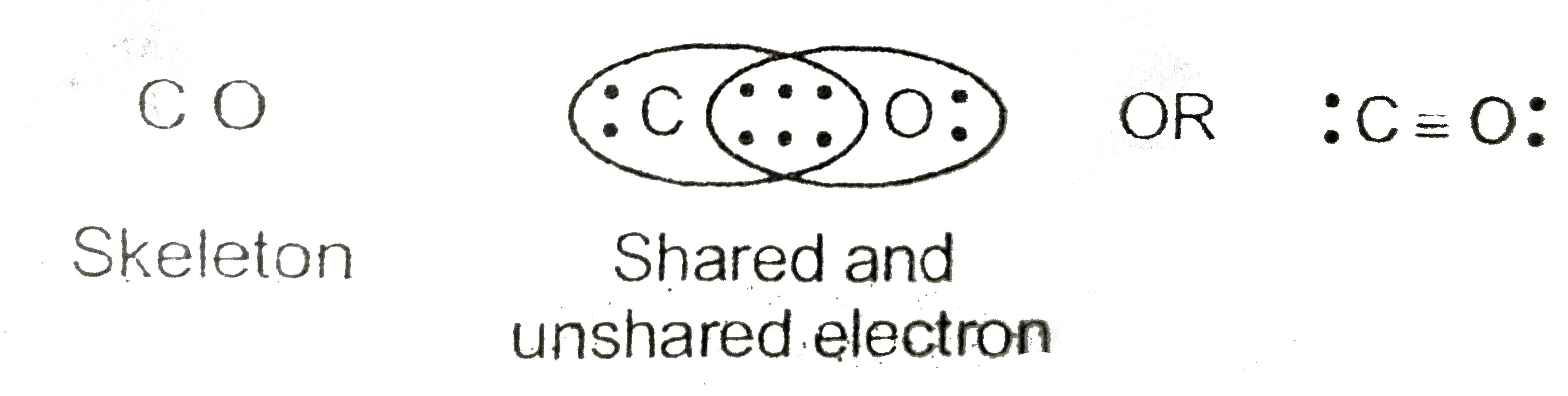
Lewis dot diagram for co. CO2 (Carbon Dioxide) Lewis Dot Structure - Science Trends The Lewis Dot Structure is a graphical representation of how electrons are distributed around the atoms which comprise a molecule. The reason for drawing/creating a Lewis dot structure is that it helps one predict the kinds of bonds, as well as a number of bonds, that can be formed around an atom. Lewis structures can be utilized to make ... PDF Lewis Dot Structures Pogil Key - Hudson City School District Complete the Lewis dot structure for IN2 Complete the Lewis dot structure for C02 10. Complete the Lewis dot structure for CO 11. Complete the Lewis dot structure for HCN STOP Your group will check your answers with the instructor before moving on. Model V: Resonance Structures— When One Lewis Structure Isn't Enough Read This! What intermolecular forces are present in CO_2? | Socratic May 06, 2018 · Dispersion Forces CO_2 has dispersion forces or van der waals forces as its only intermolecular force. Since CO_2 is made of one carbon and 2 oxygen and both carbon and oxygen are non-metals, it also have covalent bonds. For extra information, there are 3 types of intermolecular forces. Dispersion Forces Dipole-dipole Hydrogen bonds Dispersion forces are weaker than dipole-dipole and dipole ... Write the Lewis dot structure of CO molecule . - Sarthaks ... The skeletal structure of CO is written as: C O Step 3. Draw a single bond (one shared electron pair) between C and O and complete the octet on O, the remaining two electrons are the lone pair on C.
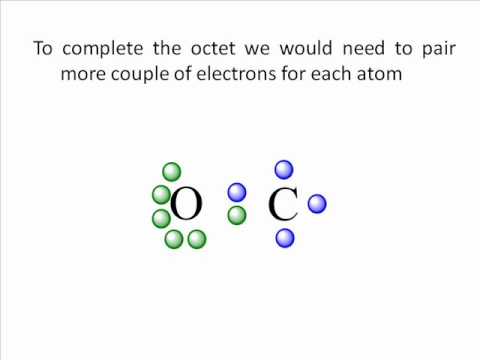
CO2 Lewis Structure | Lewis Dot Structure For Molecules ... Before we discuss the CO 2 lewis structure or lewis dot structure for CO2, we need to know the basics of lewis dot structure.Lewis dot structure works on the octet rule, which means that all the atoms would have eight electrons in their valence shell except hydrogen. CO2 Lewis Structure (2021 UPDATED) All You Need To Know 6 Steps on How to Draw CO2's Lewis Structure Calculate the total valence electrons found in a molecule. Carbon Valence Electron=4 Oxygen Valence electrons: 6*2 = 12 Total number of valence electrons = 16 Find the central atom, which is usually the one with the highest bonding sites, is the Carbon atom. Draw four dots around it. How to Draw the Lewis Dot Diagram for Carbon monoxide (CO ... te them around the central atom with the goal of filling the outer shells of each atom.In the Lewis structure of CO structure there are a total of 10 valence... Write the Lewis dot structure of CO molecule class 11 ... Let us now write the electron dot structure also called as Lewis dot structure for C O molecule. - Lewis dot structure or electron dot structure or sometimes also called as Lewis electron dot structure is the diagram which shows the bonding between the atoms of a molecule and the lone pair of electrons that may exist in the molecule.
Lewis Dot Structure Practice Worksheet With Answers - My ... A periodic table will be available for the exam but the list of rules will. Some of the worksheets for this concept are lewis dot structures and molecule geometries work lewis electron dot structure answers lewis dot structure answer key lewis structures practice work work 14 work 13 d epa rtm ent of che m istry u niversity of t exa s at atomic protons neutrons electrons. Assign an oxidation number to each element in ... - Brainly.com Oct 24, 2017 · In CH3OH - C has an oxidation number of-3, the oxidation number of O is -2 while the oxidation number of H is +1.. The oxidation number of an atom in a compound is a charge that it appears to have as determined by some arbitrarily rules. Lewis Structures: Learn How to Draw Lewis Structures ... How to Draw a Lewis Dot Structure Step 1. Determine the total number of valence electrons to be depicted in the Lewis diagram. Example: CO 2 Total = 16 Step 2. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Step 3. Determine how many electrons must be added to central element. CHEMICAL BONDING - National Institute of Open Schooling suggesting the process of chemical bonding Lewis provided a very convenient way of representing bonding in simple molecules. This is called Lewis electron-dot structures or simply Lewis structures. In Lewis structure each element is represented by a Lewis symbol. This symbol consists
Lewis Dot Structures: Definition, Structure and Sample ... The Lewis dot structure was named after the great American chemist Gilbert Newton Lewis.If the Molecular formula of a compound is known, one can draw its electron dot structure or lewis dot structure and can define the nature and position of its bond and molecules respectively. Here we will discuss more about the concept with some solved examples and questions.
Lewis Electron Dot Structures: Steps, Examples and Limitations Lewis electron dot structures are the diagrammatic representation of covalently bonded atoms in a molecule. They also show lone pairs of electrons that may exist in the molecule. They are used in the case of covalently bonded molecules as well as coordination compounds. This system was introduced by Gilbert Lewis in 1916.
AP Chemistry 2019 Free-Response Questions - College Board , is widely used in chemical fertilizers. The complete Lewis electron-dot. diagram for the urea molecule is shown above. (a) Identify the hybridization of the valence orbitals of the carbon atom in the urea molecule. (b) Urea has a high solubility in water, due in part to its ability to form hydrogen bonds. A urea molecule and
CO Lewis structure, Hybridization, and Molecular Geometry ... CO Lewis structure, Hybridization, and Molecular Geometry (Carbon Monoxide) Carbon Monoxide is a colorless and odorless gas. This gas is less dense than the air and flammable. People know about this gas, as it can also cause poisoning. Carbon Monoxide is a toxic gas that binds with hemoglobin, which interferes with its binding with Oxygen.
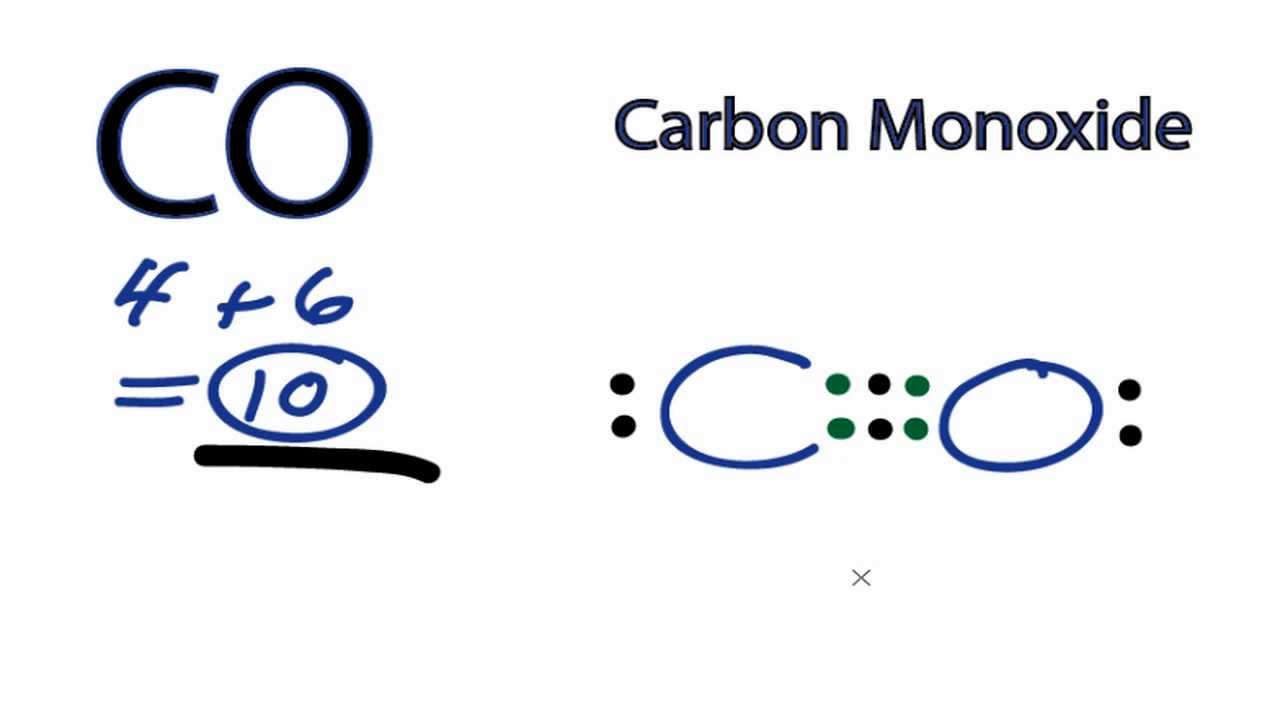
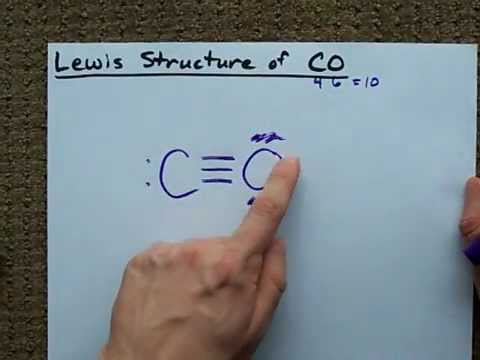
CO Lewis Structure - Lewis Dot Structure | Chem Helps CO Lewis Dot Structure To write the CO Lewis Structure, we need to understand the formation of CO. CO, Carbon Monoxide has a total of 10 valence electrons, 4 of which are C and 6 are O. It consists of a carbon and an oxygen atom. A triple bond is formed between carbon and oxygen.…
Lewis Structure of CO (Carbon Monoxide) - YouTube The Lewis Structure (Lewis Dot Diagram) for CO.1. Count electrons2. Put least electronegative atom in centre3. Put one electron pair in each bond4. Fill oute...
Lewis Dot Diagram Co2 - schemacheck.com But each oxygen in the CO2 lewis dot structure has two lone pairs. CO2 Lewis Structure | Lewis Dot Structure For Molecules. A lewis diagram helps us to know how electrons are arranged around individual atoms in a schemacheck.comal formula: CO2. Feb 19, · The Lewis Dot Structure for carbon dioxide can be represented like this: o=C=o But what ...
CO Lewis Structure, Geometry, and Hybridization ... CO Lewis Structure, Geometry, and Hybridization. Carbon monoxide (CO) is a tasteless and odorless flammable gas that is quite toxic in nature to the fauna. It is so because, carbon monoxide uses hemoglobin, an oxygen carrier, to reach throughout the body when in a concentration of more than 35ppm. The carbon monoxide is produced from the ...
Lewis Diagram For H2co - schematron.org According to schematron.org, the Lewis dot structure for H2CO, or formaldehyde, is drawn by placing a C (carbon) in the middle, putting one H (hydrogen) on either side and putting an O (oxygen) on top. This configuration contains 12 valence electrons arranged.
How can I draw the Lewis structure for CO? | Socratic Here are the steps that I follow when drawing a Lewis structure. 1. Decide which is the central atom in the structure. That will normally be the least electronegative atom (C). 2. Draw a skeleton structure in which the other atoms are single-bonded to the central atom: C-O. 3. Draw a trial structure by putting electron pairs around every atom until each gets an octet. In this editor, I will ...
Draw the Lewis structure for CO - Ask4Essay A step-by-step explanation of how to draw the Lewis Dot Diagram for CO. Use the periodic table of elements to find the total number of valence electrons for the CO molecule. Once we know how many valence electrons does carbon monoxide have we can distribute them around the central atom with the goal of filling the outer shells of each atom.
Lewis Electron Dot Structures - Detailed Explanation with ... Lewis Structure of CO (Carbon Monoxide) A carbon monoxide molecule consists of one carbon atom and one oxygen atom. The carbon atom requires four electrons to obtain octet configuration whereas the oxygen atom requires two. Therefore, the valency is satisfied via the donation of a lone pair of electrons for bonding by the oxygen atom.
PDF Lecture B1 Lewis Dot Structures and Covalent Bonding Lewis Dot Structures can be produced by following a sequence of steps. Let's produce a Lewis Dot Structure for: NH 4 + (the ammonium ion). Step 1: Count valence electrons: N = 5 4 x H = 4 x 1 = 4 "+" = -1 Total = 5+4-1= 8 electrons = 4 bonds and lone pairs. Step 2:!Arrange the atoms (identify a central atom, if possible).
Draw a lewis dot structure for Agcl and Co(NO3)2 - bartleby 8.1 Chemical Bond Formation And Lewis Electron Dot Structures 8.2 Covalent Bonding And Lewis Structures 8.3 Atom Formal Charges In Covalent Molecules And Ions 8.4 Resonance 8.5 Exceptions To The Octet Rule 8.6 Molecular Shapes 8.7 Electronegativity And Bond Polarity 8.8 Molecular Polarity 8.9 Bond Properties: Order, Length, And Dissociation ...
What do the dots on an electron dot diagram represent ... What is the Lewis dot structure for carbon monoxide? The Lewis structure for CO has 10 valence electrons. For the CO Lewis structure you'll need a triple bond between the Carbon and Oxygen atoms in order to satisfy the octets of each atom while still using the 10 valence electrons available for the CO molecule.
Lewis Structures: Dot Symbols, Diagrams, Examples - Embibe Lewis Structure of Carbon Monoxide (CO) In C O, oxygen belongs to group 16 of the Periodic Table, and carbon belongs to group 14 of the Periodic Table. Hence, oxygen has 6 valence electrons, and carbon has 4 valence electrons. Step 1- Calculating the total number of valence electrons. In C O we have- C = 4 × 1 = 4 valence electrons
How To Draw A Lewis Dot Structure For Co2 ... What Is The Lewis Diagram For Co2? The Lewis structure of carbon dioxide (CO) consists of two oxygen atoms and one carbon atom. The carbon atom in the CO has two double bonds. The valence shells of carbon atoms and oxygen atoms each contain two lone pairs. Linearity is the characteristic of CO. What Is Co2 Dot Structure?
Lewis Structure for CO - UMD The Lewis structure for CO has 10 valence electrons. For the CO Lewis structure you'll need a triple bond between the Carbon and Oxygen atoms in order to satisfy the octets of each atom while still using the 10 valence electrons available for the CO molecule. How to Draw the Lewis Dot Structure for Carbon monoxide It is helpful if you:
Lewis Dot Diagram Worksheet Answers - Worksheet Answer Key Lewis Dot Diagram Worksheet Answers - The principles of maths that a youngster researches are addition and reduction. It is difficult for a child to understand whatever at once. Educators and also parents can help him get the assistance he needs.
History of molecular theory - Wikipedia In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.. The modern concept of molecules can be traced back towards pre-scientific and Greek philosophers such as Leucippus and Democritus who argued that all the universe is composed of atoms and voids.

/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)







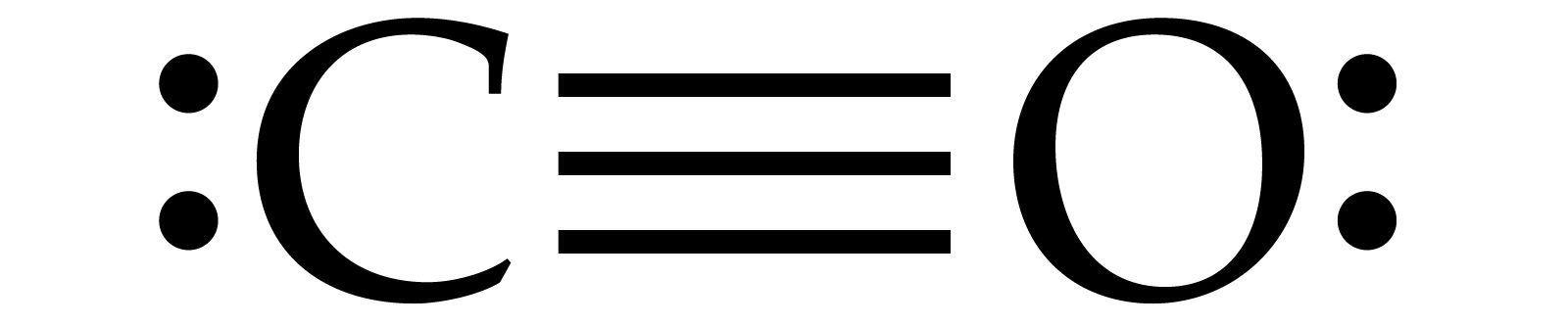



![Lewis Dot Structure of CO and [NO2]- and formal charge on each atom / chemical bonding](https://i.ytimg.com/vi/4ASNiRoJR-g/maxresdefault.jpg)


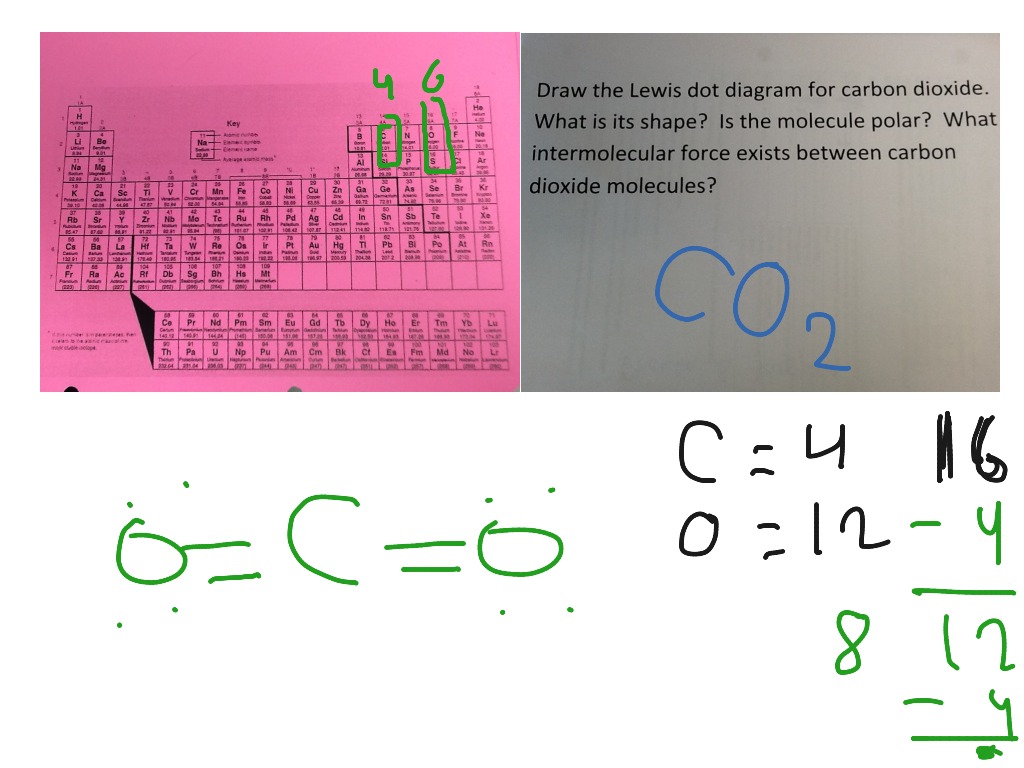



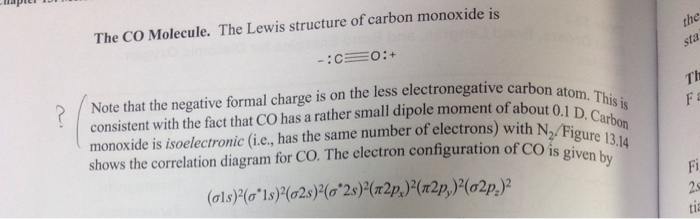

0 Response to "38 Lewis Dot Diagram For Co"
Post a Comment