38 adiabatic process pv diagram
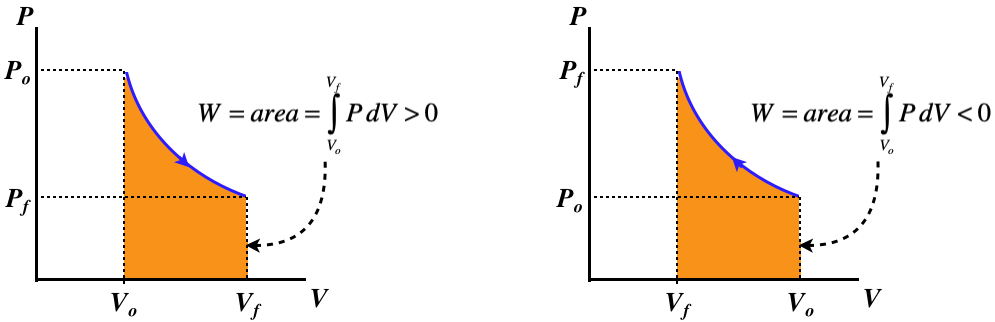
PV diagrams clearly illustrate that the work done depends on the path taken and not just the endpoints. This path dependence is seen in Figure 7a, where Both isothermal and adiabatic processes such as shown in Figure 8 are reversible in principle. A reversible process is one in which both the system... Jan 16, 2022 · Here are a number of highest rated Adiabatic Process Pv Diagram pictures on internet. We identified it from reliable source. Its submitted by dispensation in the best field. We consent this nice of Adiabatic Process Pv Diagram graphic could possibly be the most trending topic later we part it in google improvement or facebook.
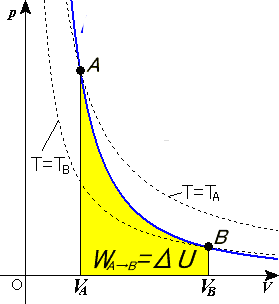
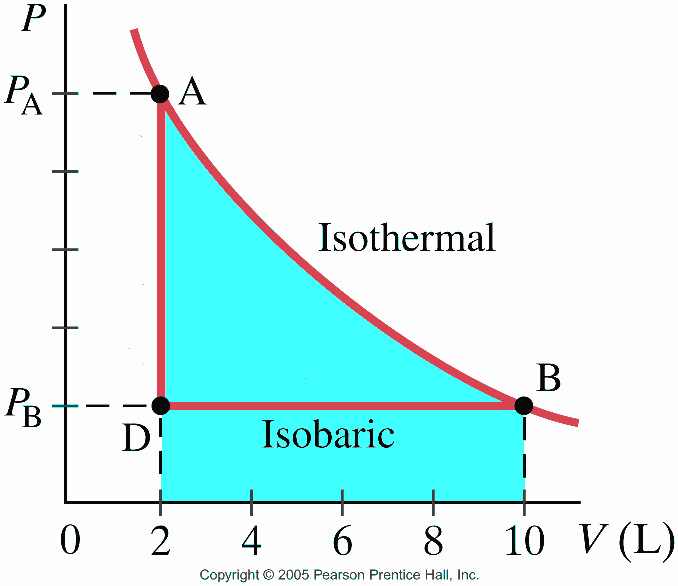
The pV-diagram for the isothermal process is shown below. Now we consider an adiabatic process, with the same starting conditions and the same final. Sketch the adiabatic processes on the p-V diagram below and compute the final temperature. (Ignore.

Adiabatic process pv diagram
The fundamental thermodynamic processes modelled on PV diagrams (isochoric, isobaric, and isothermal processes) all follow the ideal gas law except for adiabatic processes—which will be discussed in detail on its main page. The following are the examples of each process modelled on the PV diagram. File:Adiabatic-process-in-p-V-diagram.svg. From Wikimedia Commons, the free media repository. Jump to navigation Jump to search. English: Adiabatic process in p-V-diagram. Date. 23 November 2019. Adiabatic process. Computational example. Carnot cycle. Combined gas law calculator is a great tool to deal with problems related to the most common transformations of gases. Read about isobaric, isochoric, isothermal, and adiabatic processes of ideal gases...
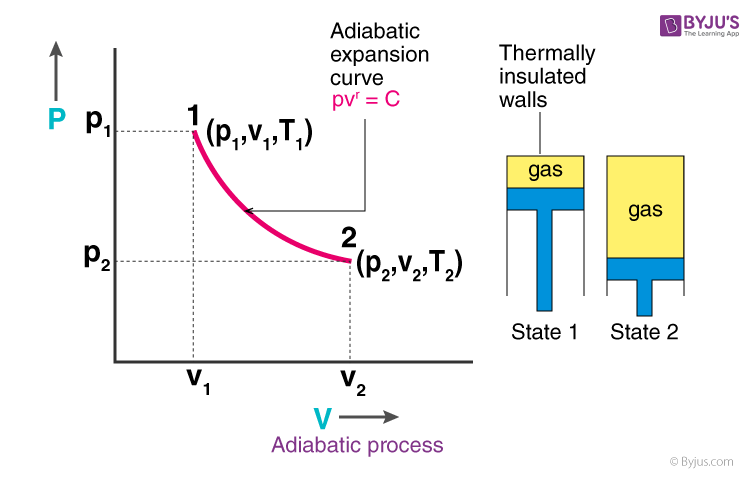
Adiabatic process pv diagram. The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. For an ideal gas, an adiabatic process is a reversible process with constant entropy. The mathematical representation of the adiabatic process is ΔQ=0 Assuming this PV diagram describes a process involving an ideal gas, we can deduce the following If we attempt to compute the entropy change for c→d by finding a point f on the PV diagram such that c→f is adiabatic and f→d is isothermal, we find that we have constructed a Carnot... PV during a polytropic process. > In an adiabatic change, the pressure and temperature of a monoatomic gas are related with relation as P∝TC, where C is equal to The process BC is adiabatic. The temperatures at A, B and C are 400K, 800K and 600K respectively. Experiment 3A: Adiabatic Gas Law Experiment 3B: Work Done by an Adiabatic Process. To show that PV/T = nR for an ideal gas and determine the value of R. Physical Principles. 3. Quickly raise the piston in an adiabatic expansion and hold up at the top of the stroke (Stroke 3 to 6 in the diagram).
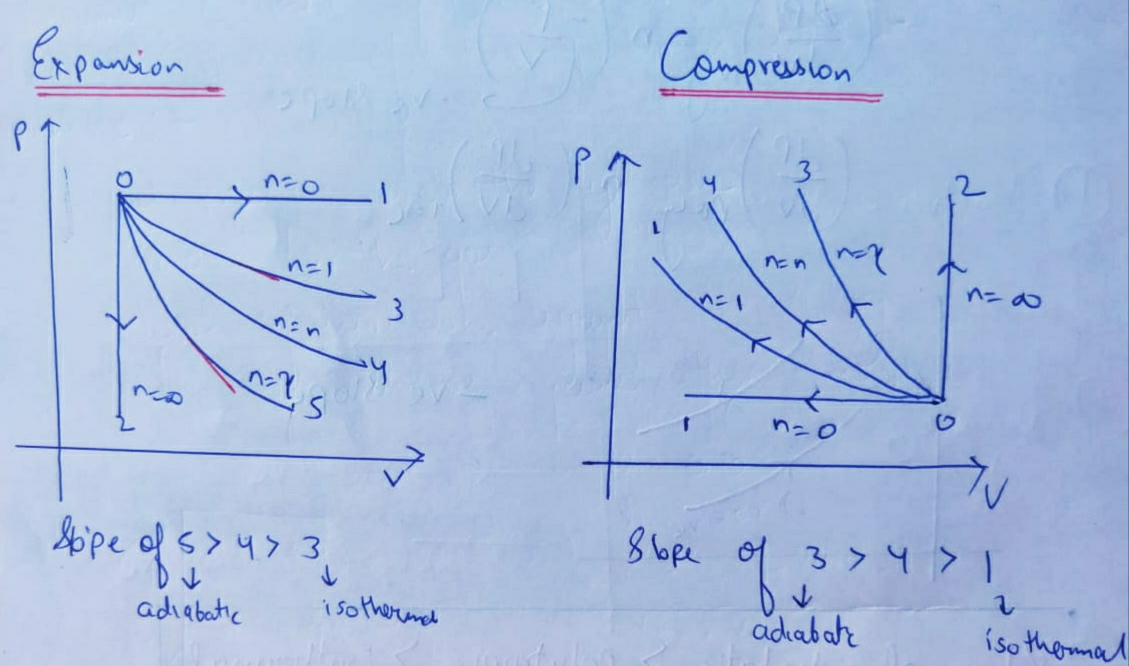
The lesson begins with the slope of an isothermal curve on PV diagram with proper graphical illustrations. Then explanation moves on to slope of adiabatic curves on PV diagram with useful mathematical equations and explanation. diagram, only adiabatic and isotherm processes have asymptotic shapes. An example case for the curves for the two processes is shown here In that case, PV is constant only for Isothermal process or in a process where there is no temperature change whatsoever as the process proceeds. Slope of Isothermal and Adiabatic Process on PV Diagram and key points about different slopes in Hindi by D Verma Sir join me ... ... PV diagrams. It explains how to calculate the work done by a gas for an isobaric process, isochoric process, isothermal process What are PV diagrams? Consider a gas sealed in a container with a tightly fitting yet movable piston as seen below. We can do work on the gas by pressing the piston downward, and we can heat up the gas by placing the container over a flame or submerging it in a bath of boiling water.
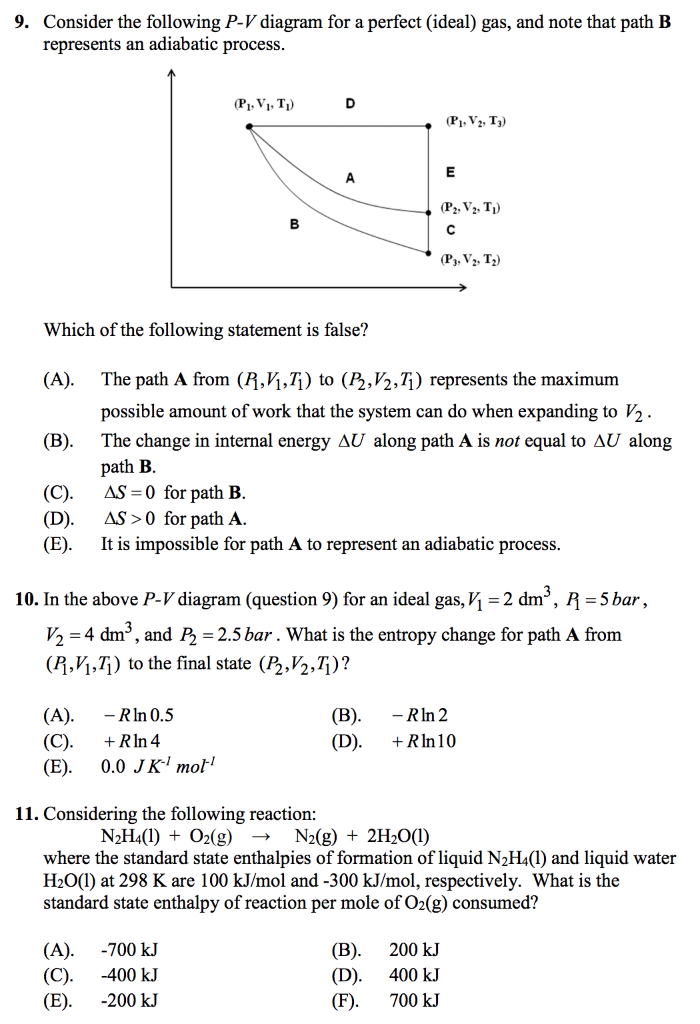
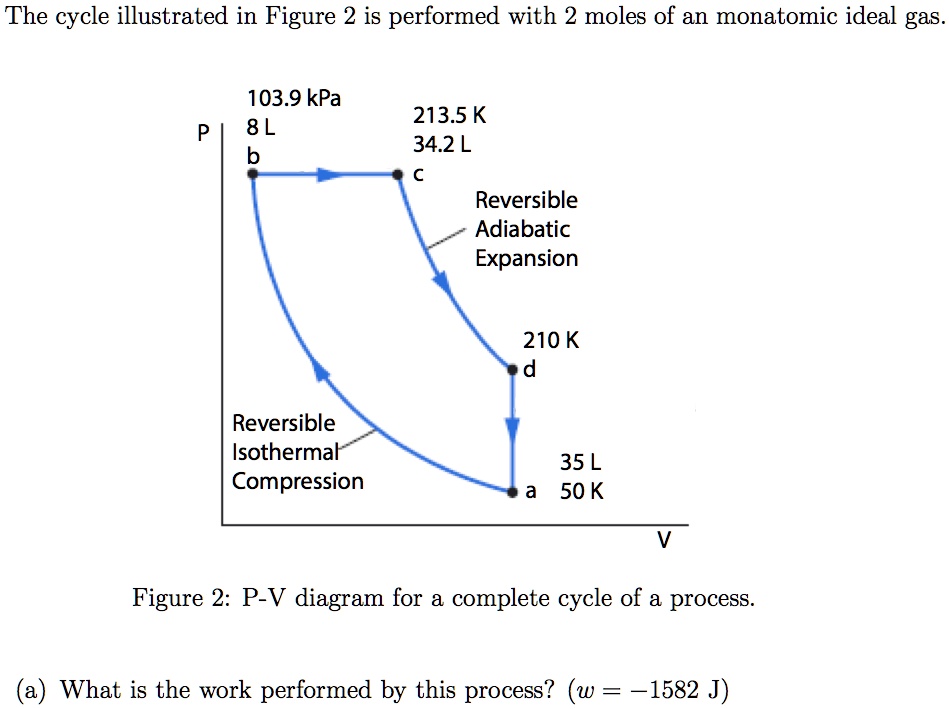
1. This question does not show any research effort; it is unclear or not useful. Bookmark this question. Show activity on this post. In non isolated systems where there is no adiabatic process, P V is constant. But the graph gets steeper in adiabatic process because of the γ over the V. Why is it there in adiabatic processes and why only over ... Another interesting adiabatic process is the free expansion of a gas. This equation is the condition that must be obeyed by an ideal gas in a quasi-static adiabatic process. Learn Adiabatic Process topic of Physics in details explained by subject experts on vedantu.com. Register free for online tutoring session to clear your Adiabatic- Reversible and Irreversible Process. There are four types of process in a thermodynamic system, which are shown via an image below During the second step the gas is compressed isothermally back to its original volume. (a) Sketch this two-step process on a pressure-volume diagram. (b) Calculate the temperature at the end of the first (adiabatic) step. (c) Calculate the work performed by the gas during the first (adiabatic) step. (d)Calculate the work performed by the gas ...
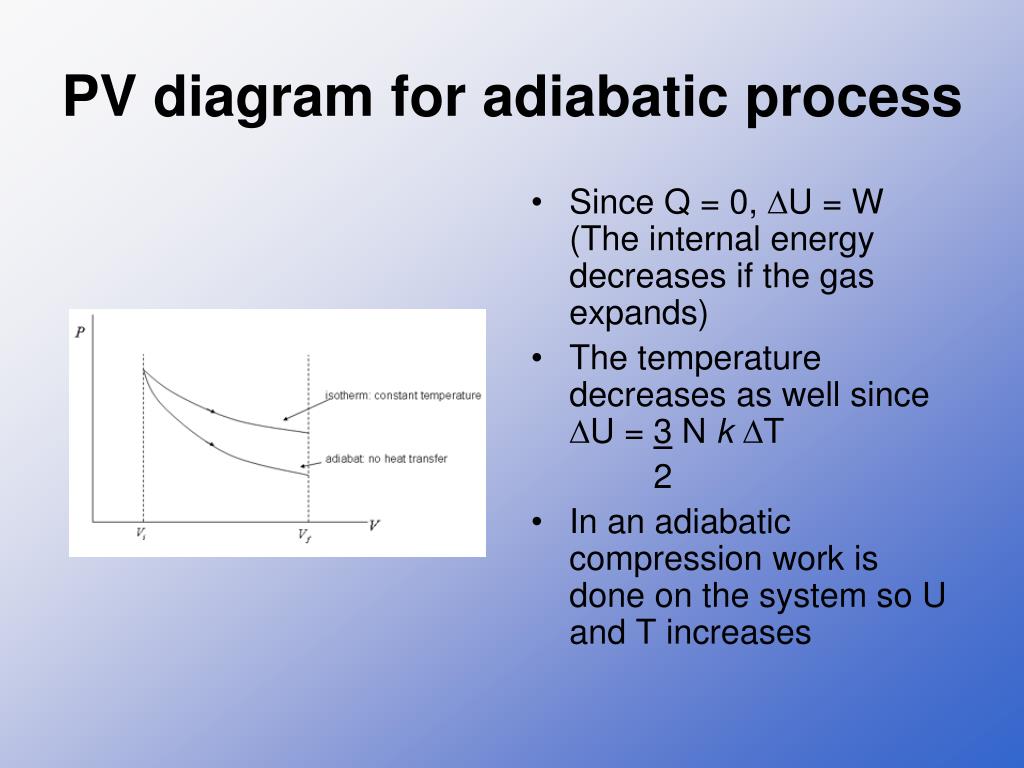
An adiabatic process is one in which no heat is gained or lost by the system. The first law of thermodynamics with Q=0 shows that all the change in internal energy is in the form of work done. This puts a constraint on the heat engine process leading to the adiabatic condition shown below.
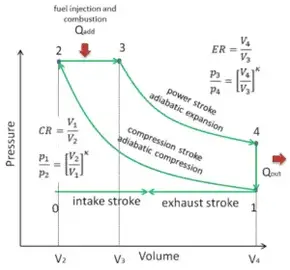
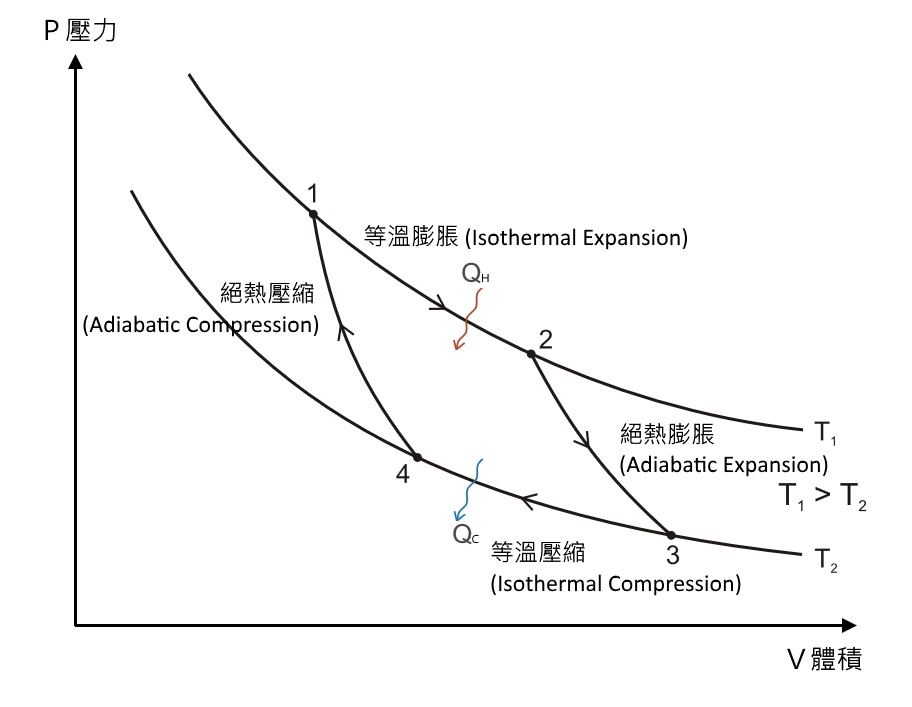
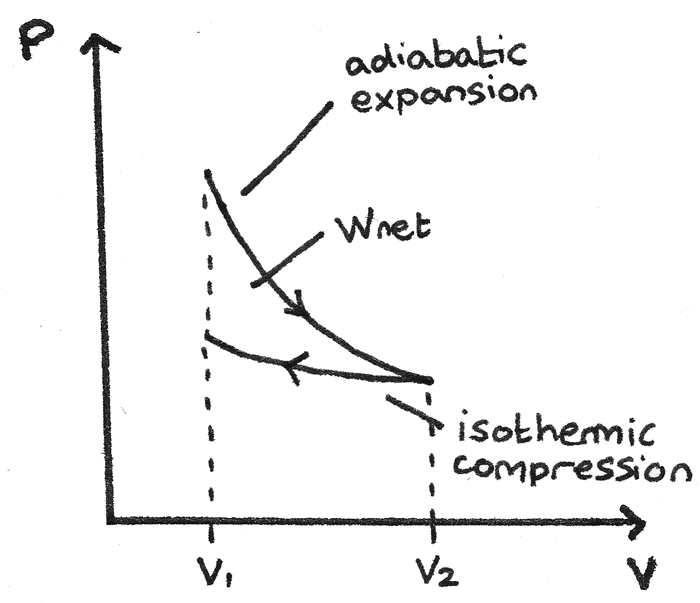
PV diagram for adiabatic expansion and adiabatic compressior. (d) Isochoric process : (a) increased pressure and.
look at PV diagrams. A PV diagram is a graph of Pressure as a function of Volume. There are four different situations that you can expect to see shown in PV diagrams: 1. Isobaric: the gas is held at a constant pressure 2. Isochoric: the gas is held at a constant volume 3. Isothermal: the gas is held at a constant temperature 4. Adiabatic: No heat flows in or out of the gas
In adiabatic processes where there is no energy transfer in the form of heat, the following relation is derived from Eqs. An adiabatic process is a reversible constant entropy process for an ideal gas without heat transfer, following the relationship. A p - V diagram of the cycle is depicted in Fig.
Adiabatic is a Greek word in which ‘a’ means ‘not’, ‘dia’ means ‘through’ and ‘bait’ means ‘hot’.So in short adiabatic is a system that does not allow heat to pass through it. Definition: It is the thermodynamic process in which there is a change in pressure, volume, and temperature of the system, but there is no exchange of heat between the system and surroundings.
Isothermal process ,Adiabatic process, Isobaric process, Solved Example Problems for Thermodynamic Processes. From the PV diagram the area under the curve AB is more, implying that the work done in isobaric process is highest and work done in adiabatic process is least.
The classical Carnot heat engine. Category. v. t. e. In thermodynamics, an adiabatic process (Greek: adiábatos, "impassable") is a type of thermodynamic process that occurs without transferring heat or mass between the thermodynamic system and its environment.
Adiabatic process is a process in which there is no exchange of heat takes place from the working substance to the surrounding during its expansion or In the P-V diagram that is shown above, a gas in the piston cylinder assembly is heated adiabatically (i.e. not heat leaves or enters into the gas.
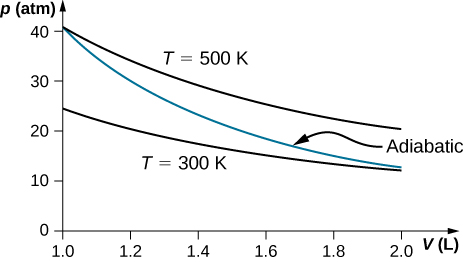
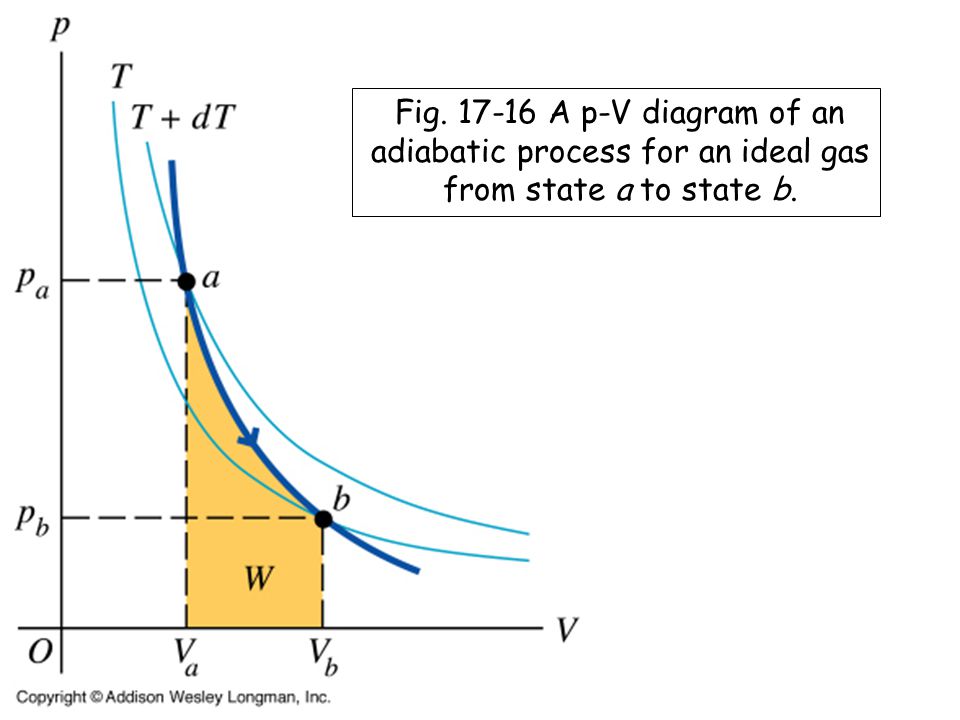
The adiabatic condition of (Figure) can be written in terms of other pairs of thermodynamic variables by combining it with the ideal gas law. In doing this, we find that. and. A reversible adiabatic expansion of an ideal gas is represented on the pV diagram of (Figure). The slope of the curve at any point is.
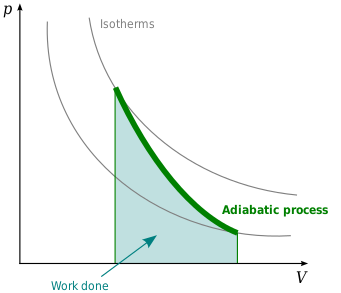
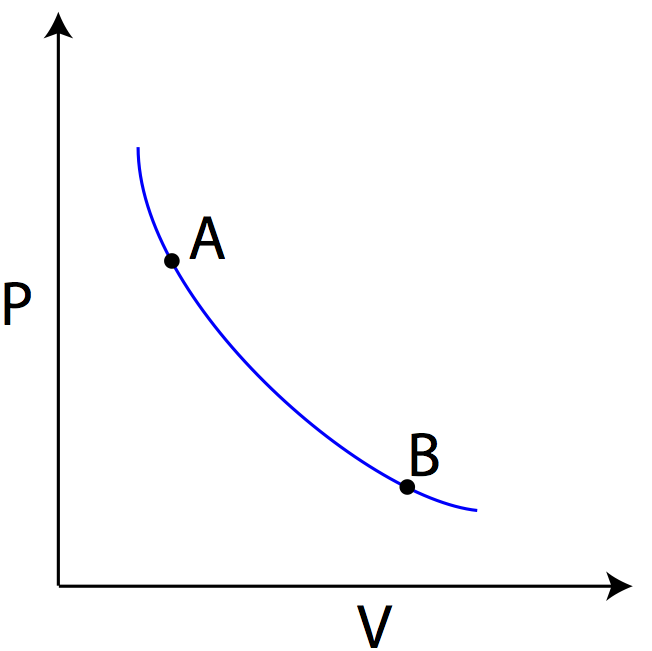
Adiabatic. Figure 1: The purple line is an example of an adiabat on a PV diagram. Adiabatic refers to a process in which no heat is transferred into or out of a system , and the change in internal energy is only done by work .
Draw the PV diagram for the adiabatic process. Tamil Nadu Board of Secondary Education HSC Science Class 11th. Textbook Solutions 9728. Important Solutions 1. Question Bank Solutions 5471. Concept Notes 472. Syllabus. Advertisement Remove all ads. Draw the PV diagram for the adiabatic process. ...
For an adiabatic process of ideal gas equation we have $PV^{\gamma} = K$ Where Calculate the work done in the process? Given $4^{1.4} =6.96$ Solution For an adiabatic Process $PV Question 2 Consider a P-V diagram in which the path followed by one mole of perfect gas in a cylindrical...
2.11 Adiabatic changes - Poisson equations. The next sections will discuss the theoretical background for describing experiments performed under various specific boundary conditions. These boundary conditions are often a significant simplification for the real experimental boundary conditions but...
The First Law is true for every process, ΔU = Q + W, the work done on the gas, but for an adiabatic process, there is no heat, so that just means ΔU equals the work done on the gas, that's the only way you're gonna add energy to the gas is by doing work on the gas or allowing the gas to do work, then energy can be removed, but you can't add or take away energy thermally, conductively, through the walls of the container.
The adiabatic process represents a state change within a system where there is no heat exchanged. Therefore, Q is equal to 0 and it is known that the change pv diagram, pressure, volume, adiabatic, process, adiabat, isometric, isothermal, isotherm, isobaric, isobar, isochoric, work, heat, first law of...
Adiabatic. Comparing isothermal and adiabatic processes. In this simulation, you can look at the difference between a constant temperature (isothermal) process and an adiabatic process. The paths look somewhat similar on the P-V diagram, but you should notice clear differences. Note that an isothermal process has no change in temperature, so the change in internal energy is zero, but in an adiabatic process the heat transferred is zero.
pV γ pVγ 1 constant 2 2 1 1 1 = = T Vγ− T V γ− pV =nRT During an adiabatic expansion process, the reduction of the internal energy is used by the system to do work on the environment. During an adiabatic compression process, the environment does work on the system and increases the internal energy. Ideal gas: adiabatic process (contd)
PV diagrams - part 2: Isothermal, isometric, adiabatic processes | MCAT | Khan Academy.
Adiabatic process. Computational example. Carnot cycle. Combined gas law calculator is a great tool to deal with problems related to the most common transformations of gases. Read about isobaric, isochoric, isothermal, and adiabatic processes of ideal gases...
File:Adiabatic-process-in-p-V-diagram.svg. From Wikimedia Commons, the free media repository. Jump to navigation Jump to search. English: Adiabatic process in p-V-diagram. Date. 23 November 2019.
The fundamental thermodynamic processes modelled on PV diagrams (isochoric, isobaric, and isothermal processes) all follow the ideal gas law except for adiabatic processes—which will be discussed in detail on its main page. The following are the examples of each process modelled on the PV diagram.






![PDF] Irreversible Adiabatic Compression of an Ideal Gas ...](https://d3i71xaburhd42.cloudfront.net/236d318ad68ed038c489652db7f01834f7a4cdf6/3-Figure2-1.png)







0 Response to "38 adiabatic process pv diagram"
Post a Comment