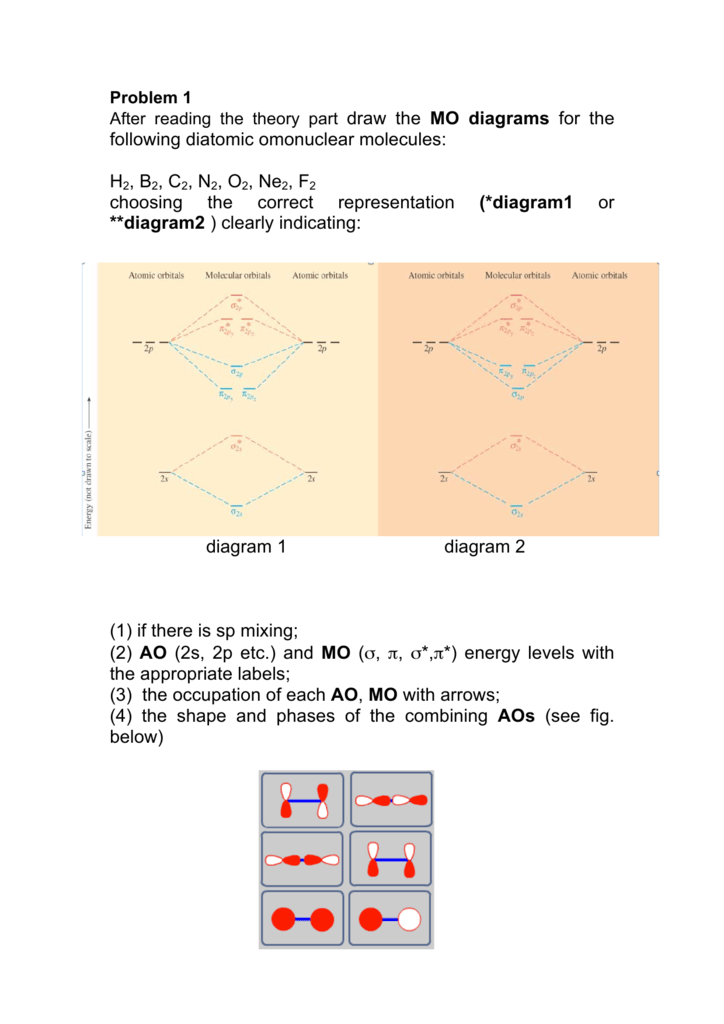
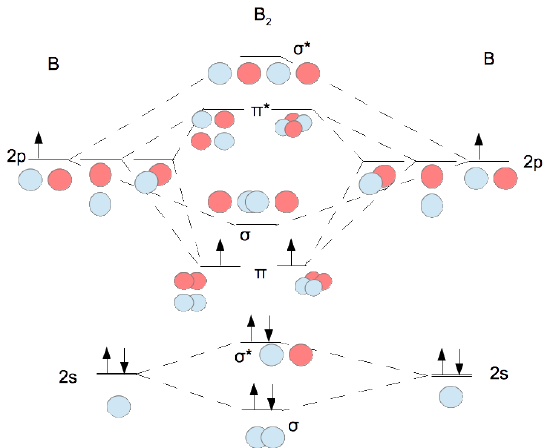
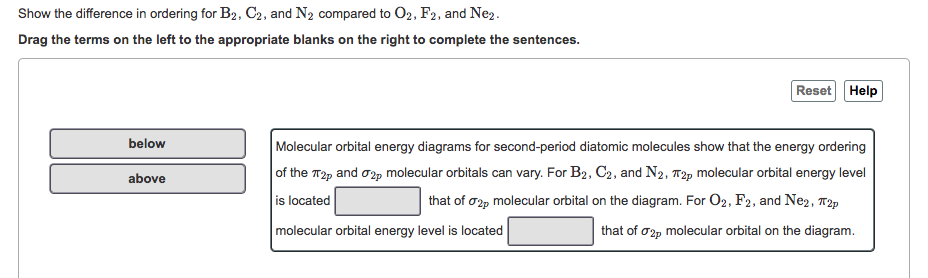
40 the mo diagram below is appropriate for b2. based on this diagram, b2
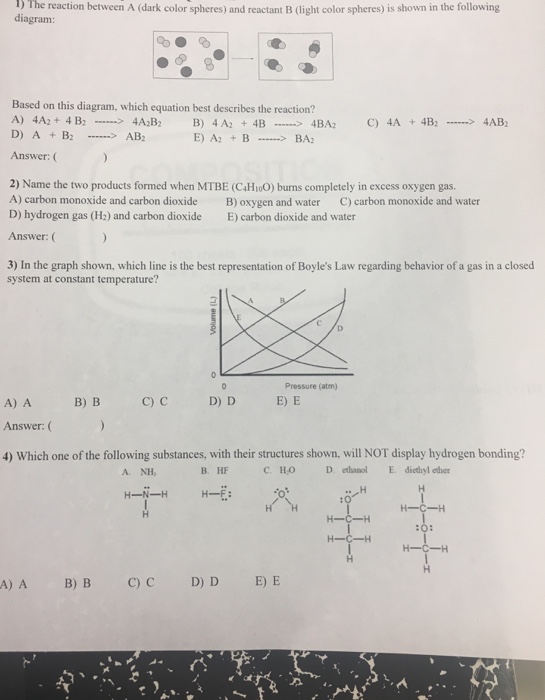
27) The MO diagram below is appropriate for B2. Based on this diagram, B2 A) has a bond order of one and is paramagnetic. B) has a bond order of one and is diamagnetic. C) has a bond order of two and is diamagnetic. D) has a bond order of two and is paramagnetic. 4 Problem Details. Which of the following is paramagnetic? (use the molecular orbital diagram) a) Li 2. b) Be 2. c) B 2. d) C 2. e) N 2. Learn this topic by watching MO Theory: Homonuclear Diatomic Molecules Concept Videos.
O2 and B2 O2. O2 and B2. Item 1: Part B Based on the molecular orbital diagram for NO, which of the following electronic configurations and statements are most correct? σ2s2 σ∗2s2 σ2p2 π2p4 π∗2p1; Magnetic. ... ↑↓, ↓ in the figure given below. σ*2p π*2p
The mo diagram below is appropriate for b2. based on this diagram, b2
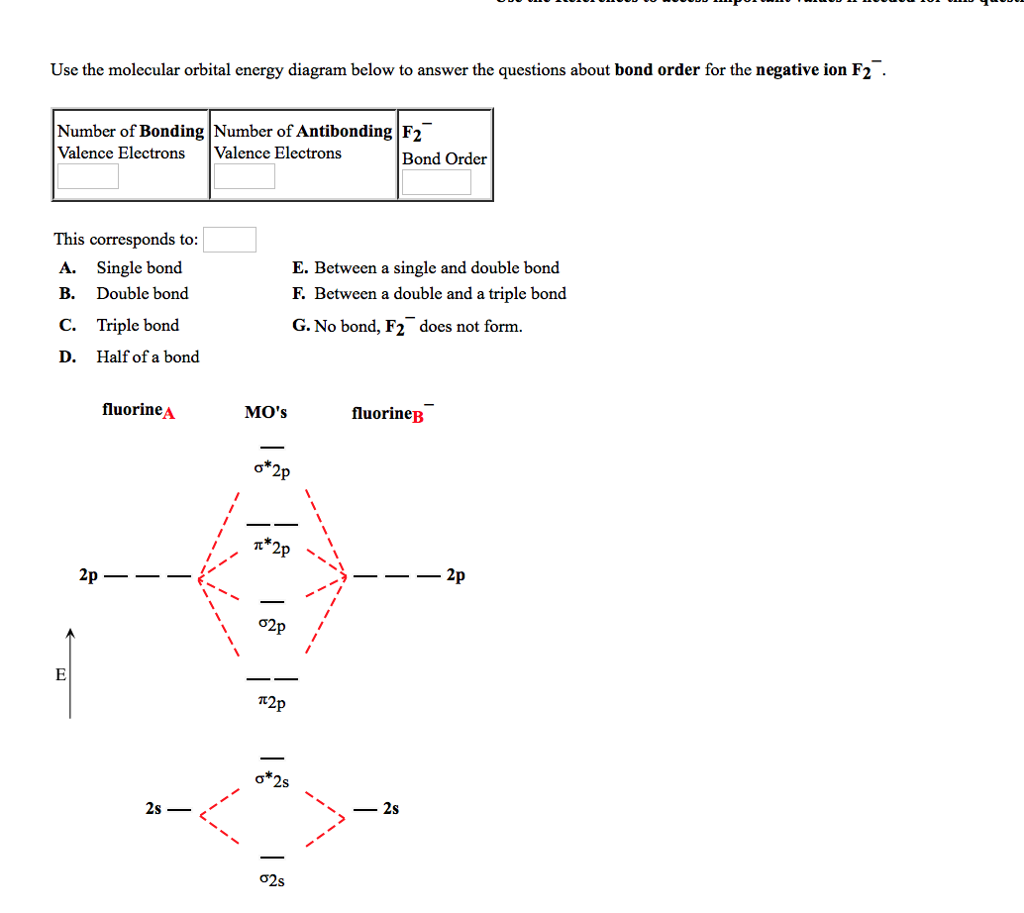
Answer (1 of 5): The atomic number of fluorine is 9, so a (neutral) F2 molecule has a total of 18 electron, or 14 valence electrons (excluding the four 1s electrons). The (F2)- ion has one more valence electron, or 15. The orbital diagram for a diatomic molecule is To find the bond order, add th... Molecular orbital energy level of Li2. diagram; the MOs go between them. Consider the MO diagram for Li2. This is a bond between two lithium atoms, which have an electron configuration of. The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital. "BO" = 1/2 Boron atom is atomic number 5 in the periodic table, so it has five electrons. Thus, B_2 carries ten total electrons. The atomic orbitals each boron contributes consists of the 1s, 2s, and 2p. The ns orbitals combine to give a portion of the molecular orbital (MO) diagram like this: where sigma^"*" indicates an antibonding sigma (sigma) MO, and sigma is the bonding MO.
The mo diagram below is appropriate for b2. based on this diagram, b2. Jan 10, 2021 — 1 Answer to 31) The following MO diagram is appropriate for Li2 and Be2. Based on this diagram, A) both are stable and diamagnetic. Bromochloromethane appears as a clear colorless liquid with a sweet chloroform-like odor.Denser than water (density 1.991 g / cm3) and insoluble in water.Hence sinks in water.Boiling point 68°C. Vapors may cause illness if inhaled. When you write the M.O. Diagram for B2, this is what you get: This shows two unpaired electrons in π2px and π2pz. However, the Bond Order of B2 = 1/2 (4-2) = 1. Doesn't this mean that B2 has a single bond between the two Boron atoms--therefore they have one sigma bond? 01/01/2017 · A composition-based definition is clear, but entropy-based definitions have conceptual problems and a range of derivative definitions are also used. These definitions fuel an unproductive controversy and distract attention from the potential scientific and practical benefits offered by the vastness of hyper-dimensional composition space. As a practical approach, the …
Problem 1. (Katz, problem 8.13) A finite state machine has one input and one output. The output becomes 1 and remains 1 thereafter when at least two 0's and two 1's have occurred as inputs, regardless of the order of appearance. 2) Use molecular orbital diagrams to determine which of the following are paramagnetic. A) O2^2-B) Ne2^2+ C) O2^2+ D) F2^2+ E) None of the above are paramagnetic; 3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic. A) B2^2+ B) B2^2-C) N2^2+ D) C2^2-E) B2 3) 10 points The MO diagram below is appropriate for B2. Based on this diagram, a) draw the molecular orbital filling diagram for B2 b) what is the bond order of B2? c) is B2 diamagnetic or paramagnetic? π2p σ2 s 4) 15 points Calculate the lattice energy for MgO (s) using a Born-Haber cycle and the fol information: Net eneroy change is 6019. Structural Analysis by R C Hibbeler 8th edition
As shown in the diagram below, the contribution to the electron density at a specific atom from a single electron in the BMO is given by the square of the coefficient of that atom in the BMO. For atoms 1 and 3, that is 1/4; for atom 2 it is 1/2. Since there are two electrons in . this MO, we must double this, giving 1/2 for C1 and C3 and 1.0 ... This repository provides a source for interatomic potentials (force fields), related files, and evaluation tools to help researchers obtain interatomic models and judge their quality and applicability. Users are encouraged to download and use interatomic potentials, with proper acknowledgement, and developers are welcome to contribute potentials for inclusion. ex3 MO supplement Diagram 9‐1 The molecular orbital diagram below may be used for the following problem(s). For oxygen and fluorine, the σ2p orbital should be lower in energy than the π2p. However, the diagram will still yield correct bond order and magnetic behavior for these molecules. CMOS DIGITAL INTEGRATED CIRCUITS BY SUNG MO KANG & YUSUF LEBLEBICI(prince367) Chaitanya Reddy. Download PDF. Download Full PDF Package. This paper. A short summary of this paper. 37 Full PDFs related to this paper. READ PAPER. CMOS DIGITAL INTEGRATED CIRCUITS BY SUNG MO KANG & YUSUF LEBLEBICI(prince367) …
Valence Bond Model vs. Molecular Orbital Theory . Because arguments based on atomic orbitals focus on the bonds formed between valence electrons on an atom, they are often said to involve a valence-bond theory.. The valence-bond model can't adequately explain the fact that some molecules contains two equivalent bonds with a bond order between that of a single bond and a double bond.
According to molecular orbital theory, the atomic orbitals having comparable energy overlap and result in the formation of the same number of molecular orbitals ...
27/06/2017 · Read Electrical power systems ddas by gopinath on Issuu and browse thousands of other publications on our platform. Start here!
Image transcriptions Question ( 3 ) solution a) following are the construction of molecular orbital diagram for B2 molecule . B ( Is 2 2 5 2 2 pl ) + B ( 15 2 2 5 2 261 ) = B, ( 10 election) < is molecular orbital diagram for B , is as shown below . 2P 2p 2s 2 5 I's Energy. 515 IS Atomic orbital Is Atomic orbital of (B ) Molecular of 'B' orbital of B 2 1i ) The electronic configuration of the ...
Figure 8.35 The molecular orbital energy diagram predicts that H2 will be a stable molecule with lower energy than the separated atoms. A dihydrogen molecule contains two bonding electrons and no antibonding electrons so we have. bond order in H 2 = ( 2 − 0) 2 = 1. bond order in H 2 = ( 2 − 0) 2 = 1.
This repository provides a source for interatomic potentials (force fields), related files, and evaluation tools to help researchers obtain interatomic models and judge their quality and applicability. Users are encouraged to download and use interatomic potentials, with proper acknowledgement, and developers are welcome to contribute potentials for inclusion.
Draw the molecular orbital diagram for B 2. The number of unpaired electrons in the B 2 molecule is _____. (a) zero (b) 1 (c) 2 (d) 3 (e) 4 8. Which one of the following statements is false? (a) Valence bond theory and molecular orbital theory can be described as two different views of the same thing.
draw the molecular orbital diagram for B2. the number of electrons in the pi2p molecular orbital is. 1. ... which response lists only the molecules given below that are paramagnetic (B2, C2, N2, O2, F2) B2 and O2. which response lists all the following diatomic molecules and ions that are paramagnetic (Be2, B2, B2+2, C2+2, C2-2, O2-, O2-2)
Answer (1 of 5): NO+ has 10 valence electrons: (Sigma2s)^2(Sigma*2s)^2(Pi2px,Pi2py)^4(Sigma2pz)^2 NO has 11 valence electrons: Same as NO+ but add (Pi*2px,Pi*2py)^1 NO- has 12 valence electrons: Same as NO but change ^1 at the end to ^2. Your MO diagram for NO should look like this: O is m...
Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
19. Refer to the MO Diagrams. Use molecular orbital theory to determine if the molecular are paramagnetic or diamagnetic. c. Li2 d. N2 a. F2 20. Refer to the MO Diagrams. Assuming that the molecular orbital energy diagram for a homonuclear diatomic molecule applies to a hero nuclear diatomic molecule, determine bond order for each below. c. NO ...
The MO diagram below is appropriate for B2. Based on this diagram, B2. has a bond order of one and is paramagnetic. The following MO diagram is appropriate for Li2 and Be2. Based on this diagram, Li2 is stable and diamagnetic, but Be2 is unstable.
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ...
Diatomic Species by Molecular Orbital Theory. Even rather simple molecular orbital (MO) theory can be used to predict which homonuclear diatomic species - H 2, N 2, O 2, etc. - will exist, explain many properties - for example why O 2 is a paramagnetic diradical - and identify the important frontier molecular orbitals (FMOs).
In the diagram below, we show the ve state identi ers in the top half of each circle, and the associated values of output L in the bottom of each circle. Note that only the nal state shows the device as being unlocked. We transition from one state to the next based on input values B1 and B2. It is often easiest to start a nite state diagram
18/12/2021 · High-performance structural materials are critical to the development of transportation, energy, and aerospace. In recent years, newly developed high-entropy alloys with a single-phase solid-solution structure have attracted wide attention from researchers due to their excellent properties. However, this new material also has inevitable shortcomings, such as …
Get 24⁄7 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply.
Molecular Orbital (MO) Theory helps us to explain and understand certain Part B - Molecular Orbital Energy Diagrams & Bond Order . + and Be2.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic ...
The bond consists of two electron clouds which lie above and below the plane of carbon and hydrogen atoms. (a) Formation of ethylene (b) Molecular orbital structure molecule of ethylene Thus, ethylene molecule consists of four sigma C - H bonds, one sigma C - C bond and one bond between carbon-carbon atom. The bond length of carbon-carbon ...
From Molecular Orbital Diagram, which is most stable? A. F2 2-B. Ne2 2+ C. O2 2+ D. F2 E. F2 2+ C. O2 2+ Choose the compound below that should have the highest melting point according to the ionic bonding model. A. CaS B. NaCl C. RbI D. MgO E. AlN. E. AlN. From Molecular Orbital Diagram, which is most stable? A. C2 2-B. B2 C. B2 2+ D. N2 2+ E ...
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
Based on the balanced chemical equation shown below, determine the molarity of a solution containing Fe2+(aq), if 40.00 mL of the Fe2+(aq) solution is required to completely react with 30.00 mL of a 0.125 M potassium bromate, KBrO3(aq), solution.The chemical equation for …
Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy. We can ignore the 1s orbitals, because they do not contain the valence electrons. Each boron atom has one 2s and three 2p valence orbitals. The 2s orbitals will overlap to form 2sσ and 2sσ ...
Draw the MO diagram for the B2 molecule and answer the following questions: Based on your MO diagram, would you expect the B2 molecule to be paramagnetic or ...4 answers · Top answer: two represent the molecular orbital Energies for B two. We recognize that B2 is one where ...
"BO" = 1/2 Boron atom is atomic number 5 in the periodic table, so it has five electrons. Thus, B_2 carries ten total electrons. The atomic orbitals each boron contributes consists of the 1s, 2s, and 2p. The ns orbitals combine to give a portion of the molecular orbital (MO) diagram like this: where sigma^"*" indicates an antibonding sigma (sigma) MO, and sigma is the bonding MO.
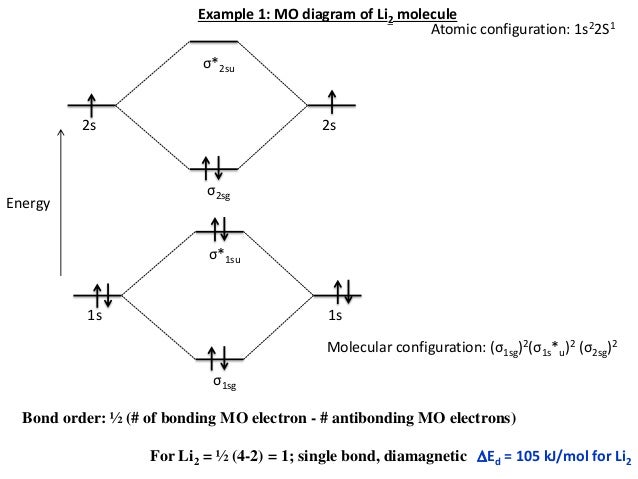
Molecular orbital energy level of Li2. diagram; the MOs go between them. Consider the MO diagram for Li2. This is a bond between two lithium atoms, which have an electron configuration of. The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital.
Answer (1 of 5): The atomic number of fluorine is 9, so a (neutral) F2 molecule has a total of 18 electron, or 14 valence electrons (excluding the four 1s electrons). The (F2)- ion has one more valence electron, or 15. The orbital diagram for a diatomic molecule is To find the bond order, add th...





















0 Response to "40 the mo diagram below is appropriate for b2. based on this diagram, b2"
Post a Comment