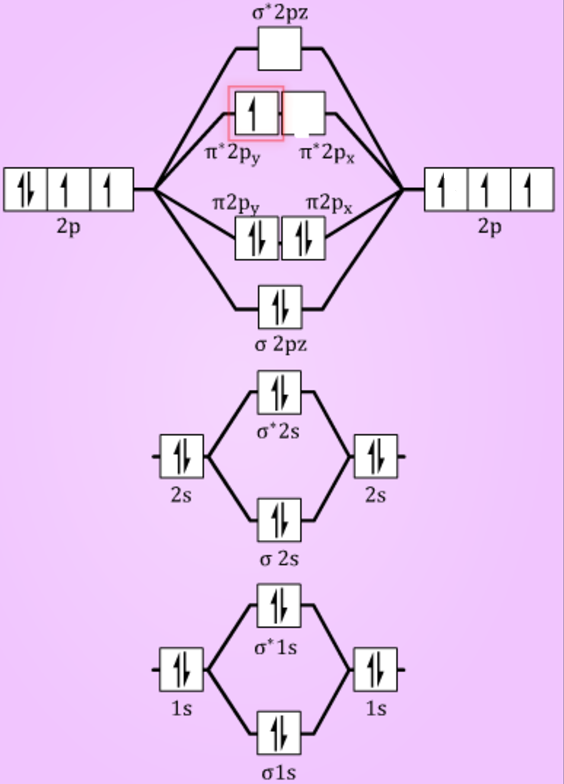
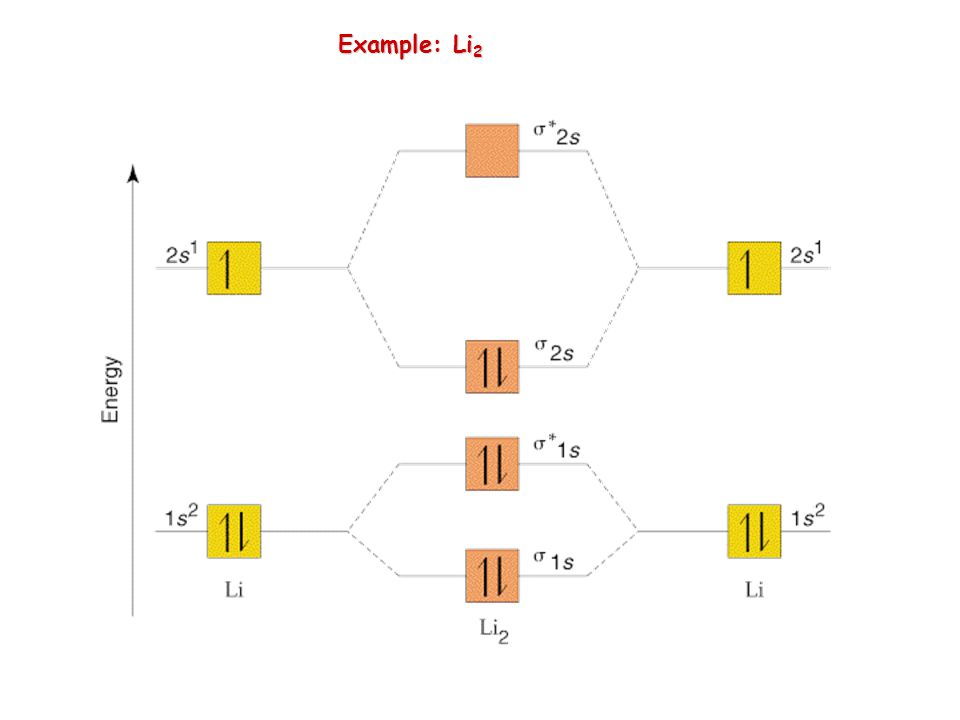
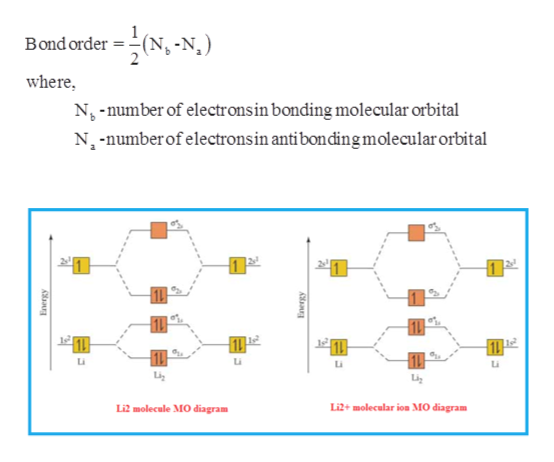
40 li2+ molecular orbital diagram
How to draw Molecular Orbital Diagram of Li2 ,Li 2+ , Li2 - | Simplest Trick - Chemistry By anumsunum on August 20, 2021 • ( 1 Comment ) Also Watch Molecular orbital diagram of O2 , O2 +2 , 02 - 2 ( in Urdu / Hindi) As per molecular orbital (MO) theory, all the constituent atoms in a molecule contribute to the formation of molecular orbitals. These MOs are a linear combination of the atomic orbitals. Thus, the electrons in a molecule are not individually assigned to atomic orbitals but to molecular orbitals. Let us have a look at the MO diagram for F2.
Dec 11, 2021 · Formation of molecular orbitals, linear combination of atomic orbitals (LCAO), conditions for combination of atomic orbitals, energy level diagrams for molecular orbitals, bonding in some homo nuclear diatomic molecules-H2, He2, Li2, B2, C2, N2, and O2
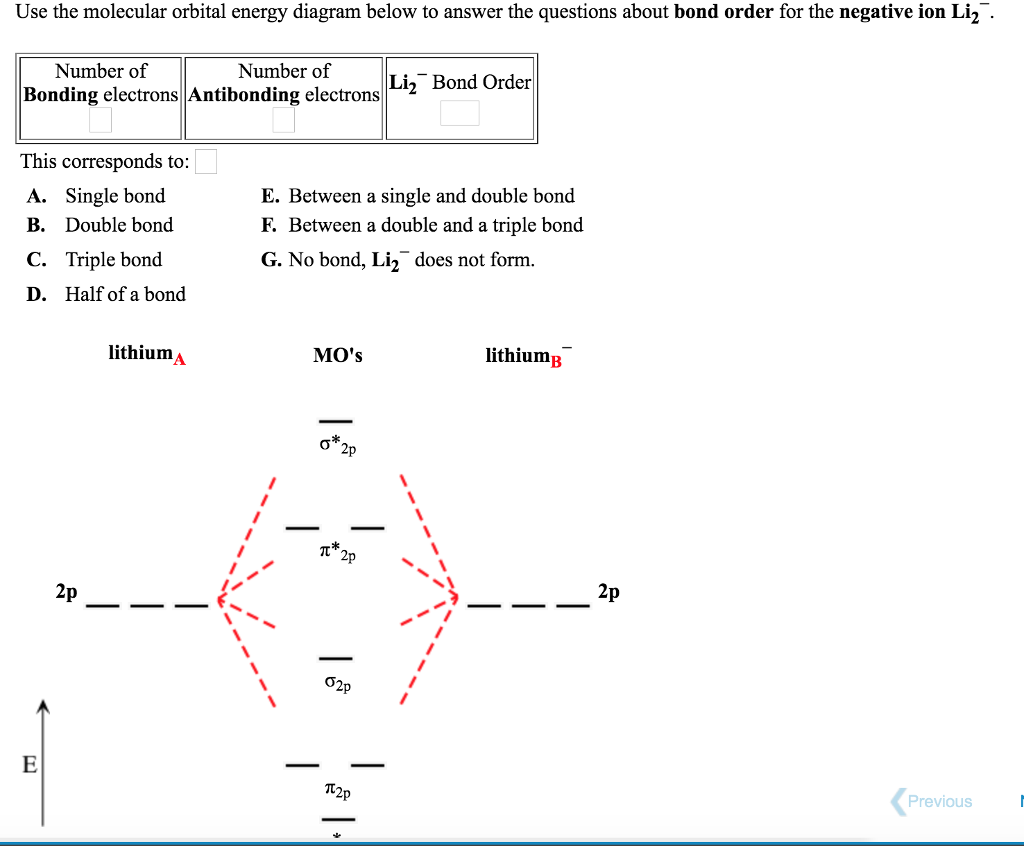
Li2+ molecular orbital diagram
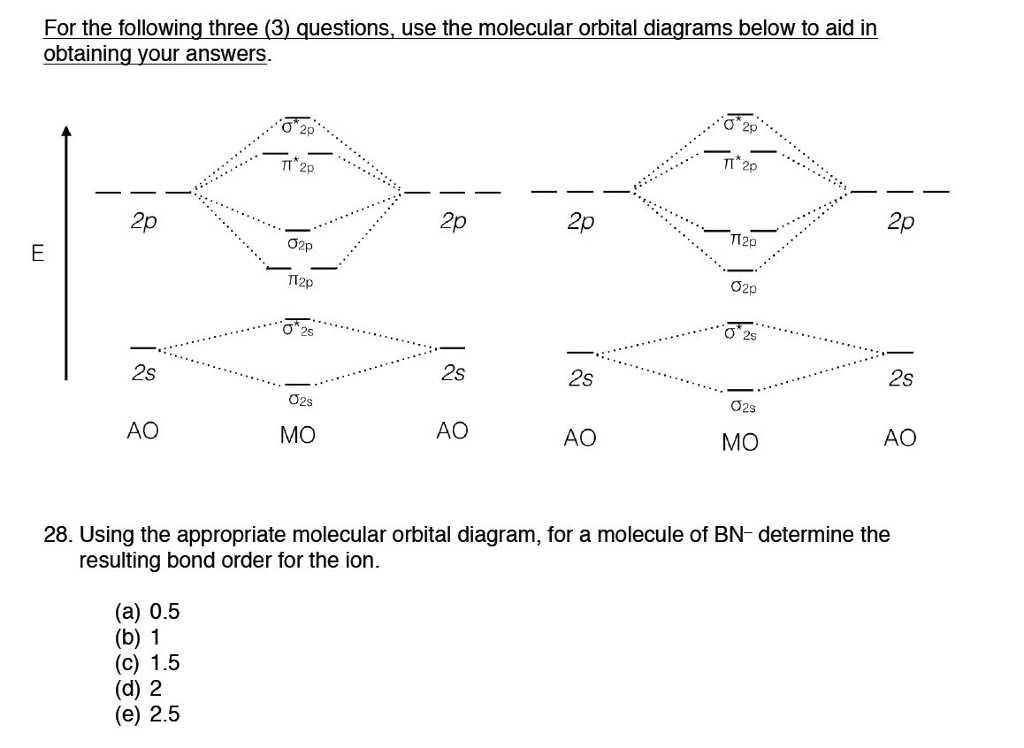
B) Li2 is stable and diamagnetic, but Be2 is unstable. C) Be2 is stable and diamagnetic, but Li2 is unstable. D) Be2 is stable and paramagnetic, but Li2 is unstable. 32) Given that O2 is paramagnetic and has a bond order of 2, and its highest occupied molecular orbital is antibonding, what would be the expected bond orders for O22- and O22+? Be shown using Lewis structures for the H3O+ Lewis structure for the hydronium! Hydronium cations a crystal structure hydronium resonance structures stoichiometric OH groups, water molecules, hydronium. Octet-Rule exception many resonance structures of these anions with protonated free acid of. Two molecular orbitals are formed after the combination of two atomic orbitals. Of these two molecular orbitals, one is a bonding molecular orbital and the other is an antibonding molecular orbital. The energy of the bonding molecular orbital is lower, and hence it has greater stability and vice versa in the case of the anti-bonding molecular ...
Li2+ molecular orbital diagram. Finally, Does Li2 have a bond order of 2?, It's going to equal to ½ times the number of electrons in the bonding molecular orbital minus the number of electrons in the antibonding molecular orbital. … So 2 - 1 is 1, and then ½ * 1 is just ½ or 0.5 so bond order for this molecule would be ½ and it's paramagnetic because it has one unpaired electron. Draw a molecular orbital diagram of N2 or O2 with magnetic … - Bond order: In simple words, It can be stated that bond order is the difference between the number of bonds and antibonds. Bond number also gives an indication of the stability of a bond. Jun 30, 2020 · So the bond order of B2 is equal to 1, which you can get by drawing the molecular orbital diagram and performing the equation Bond Order = . 5 * (# of bonding electrons - # of antibonding electrons). However, when you draw the Lewis structure of B2, you get a triple bond. Physical Chemistry a molecular approach; McQuarrie Donald A., Simon John A.; University Science Books; 1997; California
We would like to show you a description here but the site won’t allow us. Molecular Orbital Diagram For Li2 - schematron.org The molecular orbital theory is highly dependent on the geometry of the complex and can successfully be used for describing octahedral complexes, tetrahedral and square-planar complexes. The main features of molecular orbital Now for Li2 and H2 as both have same bond order we have to look at the distribution of electrons in bonding as well antibonding orbitals.In such case we observed that H2 has electrons only in bonding orbital and antibonding orbital is empty,but in Li2 molecule the electrons are in antibonding orbital also,so it will be … A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.
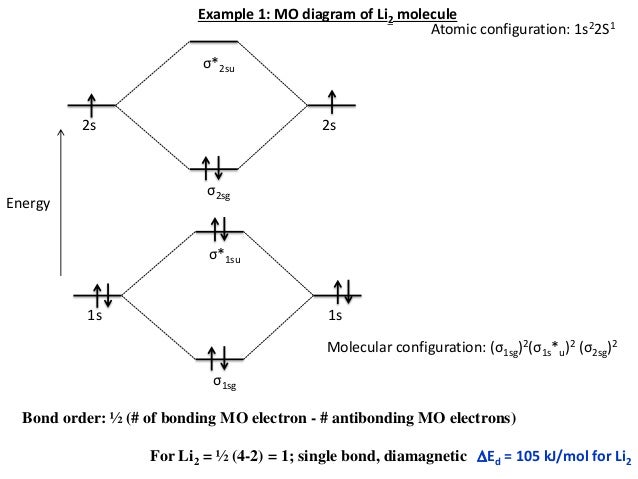
Electronic configuration of Li atom is 1s2 2s1 . How to draw Molecular Orbital Diagram of Lithium molecule (Li 2) ? According to the molecular orbital theory ,the molecular orbital diagram of lithium molecule is shown below: The 2 electrons are present in σ 2s orbitals . The anti bonding molecular orbital σ*2 s is empty . Thus Dilithium, Li2, is a strongly electrophilic, diatomic molecule comprising two lithium atoms covalently bonded together. Li2 is known in the gas phase. It has a bond order of 1, an internuclear separation of 267.3 pm and a bond energy of 102 kJ/mol or 1.06eV in each bond . This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. The valence molecular orbital diagram for Li2- is shown. THe molecular orbital bond order for this species is equal to _____ and Li2- is _____ stable than Li2 -1/2
A blank molecular orbital diagram (Part B 1 figure) has been provided to help you.Drag the formulas to the appropriate magnetic bin :C2^2+,Li2^2-,B2^2- We offer the best custom paper writing services.
Keywords: MO diagram, C22+, B22-, Li2-, molecular orbital diagram, paramagnetic, diamagnetic, paramagnetism, diamagnetism, paired electrons, unpaired electrons. Answer : For a species to be diamagnetic it needs to have no unpaired electrons and for paramagnetic the species needs t have unpaired electrons.
Two molecular orbitals are formed after the combination of two atomic orbitals. Of these two molecular orbitals, one is a bonding molecular orbital and the other is an antibonding molecular orbital. The energy of the bonding molecular orbital is lower, and hence it has greater stability and vice versa in the case of the anti-bonding molecular ...
Be shown using Lewis structures for the H3O+ Lewis structure for the hydronium! Hydronium cations a crystal structure hydronium resonance structures stoichiometric OH groups, water molecules, hydronium. Octet-Rule exception many resonance structures of these anions with protonated free acid of.
B) Li2 is stable and diamagnetic, but Be2 is unstable. C) Be2 is stable and diamagnetic, but Li2 is unstable. D) Be2 is stable and paramagnetic, but Li2 is unstable. 32) Given that O2 is paramagnetic and has a bond order of 2, and its highest occupied molecular orbital is antibonding, what would be the expected bond orders for O22- and O22+?

This photograph depicted Enteric Diseases Laboratory Branch (EDLB), Public Health scientists, as they were preparing enteric bacteria samples for “DNA fingerprintingâ€, using pulsed-field gel electrophoresis (PFGE).

Vaccine. Dr. J. Michael Hamilton preparing the carcinoembryonic antigen (CEA) vaccinia vaccine used to try to prevent cancer. He is diluting the concentrated vaccinia virus into a dose level appropriate for administration to a patient. This vaccinia marks any cancer cells expressing the CEA.
Vials of Blood. Vials of blood taken in the course of patient care at the National Institutes of Health Clinical Center in Bethesda, Maryland. Test tubes. Blood test. Creator: Daniel Sone

DNA Genotyping and Sequencing. A technician at the Cancer Genomics Research Laboratory, part of the National Cancer Institute's Division of Cancer Epidemiology and Genetics (DCEG), washes arrays used in genome-wide association studies (GWAS). These studies search the genome for small variations, called single nucleotide polymorphisms or SNPs, that occur more frequently in people with a particular disease than in people without the disease.
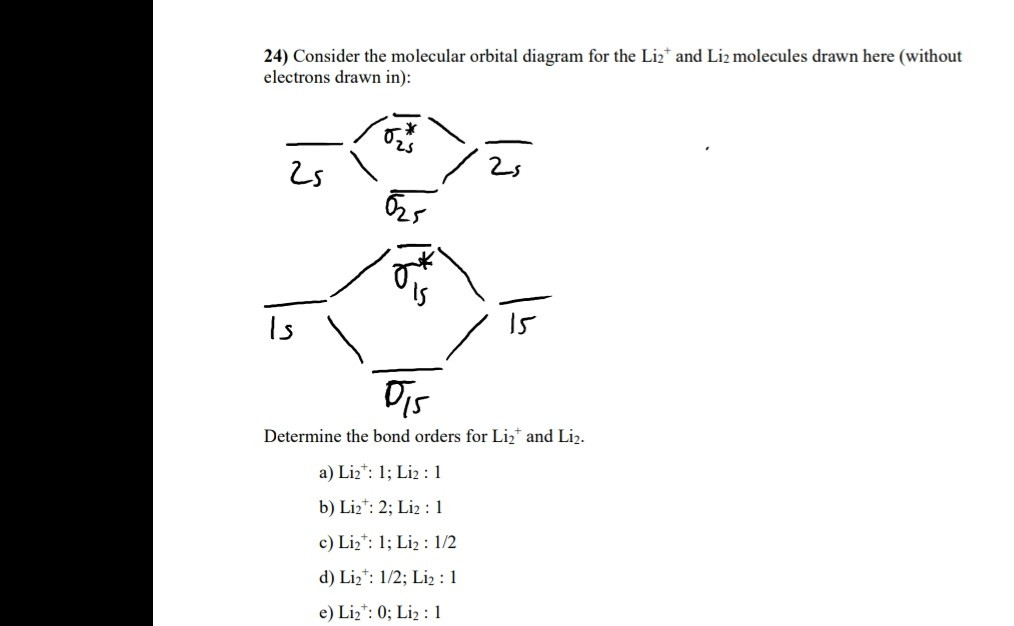











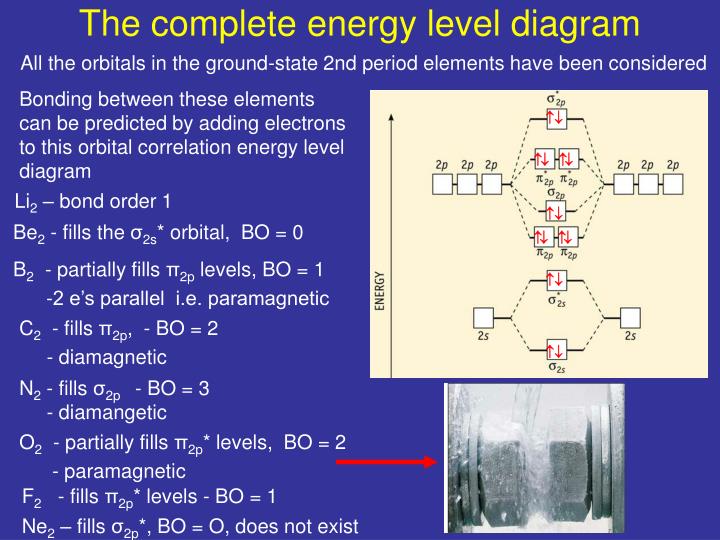



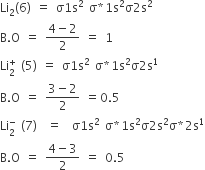


0 Response to "40 li2+ molecular orbital diagram"
Post a Comment