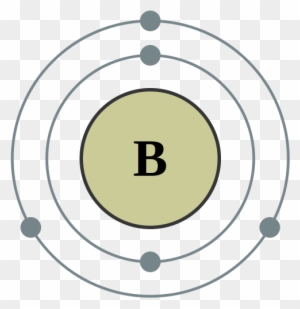
39 orbital filling diagram for boron
Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium Where are the Electrons? Write the full electron configuration, short-hand electron configuration, and fill in the orbital . Place the ending configuration and the dot diagram for the above ... Orbital filling diagrams | The Cavalcade o' Chemistry Feb 23, 2016 · The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals.
1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium
:max_bytes(150000):strip_icc()/boronatom-58b6028a5f9b5860464c90ab.jpg)
Orbital filling diagram for boron
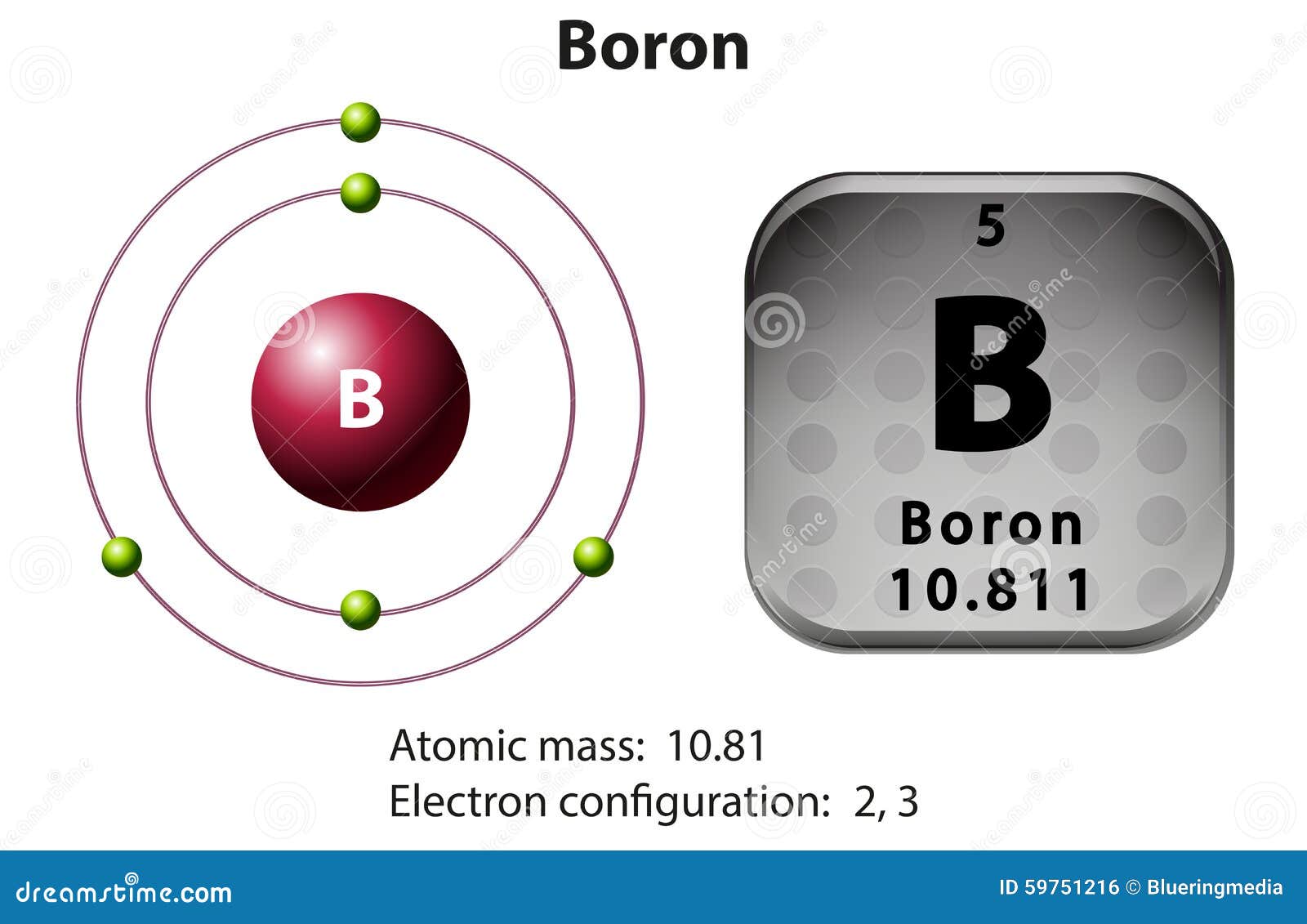
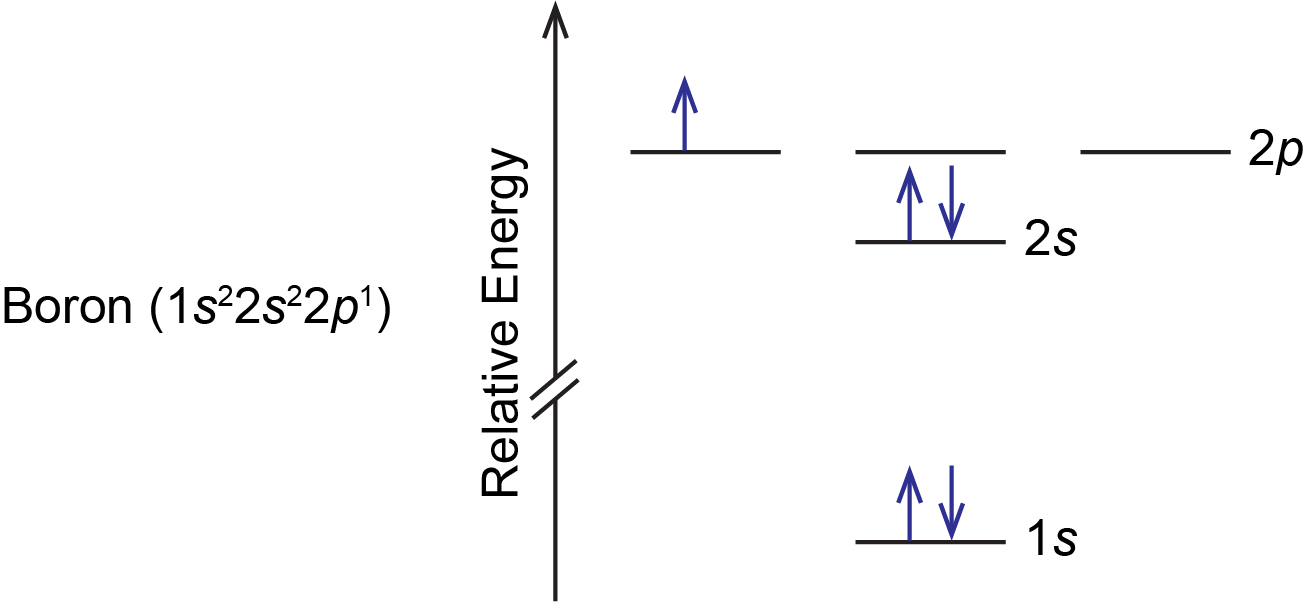
The number of unpaired electrons in an atom can be determined from the orbital filling diagrams and correctly applying Hund's rule. Boron has one unpaired electron, while carbon has two, and nitrogen has three. Boron is the fifth element with a total of 5 electrons. In writing the electron configuration for Boron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for B goes in the 2s orbital. The remaining electron will go in the 2p orbital. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. Show transcribed image text Draw an orbital diagram for boron.
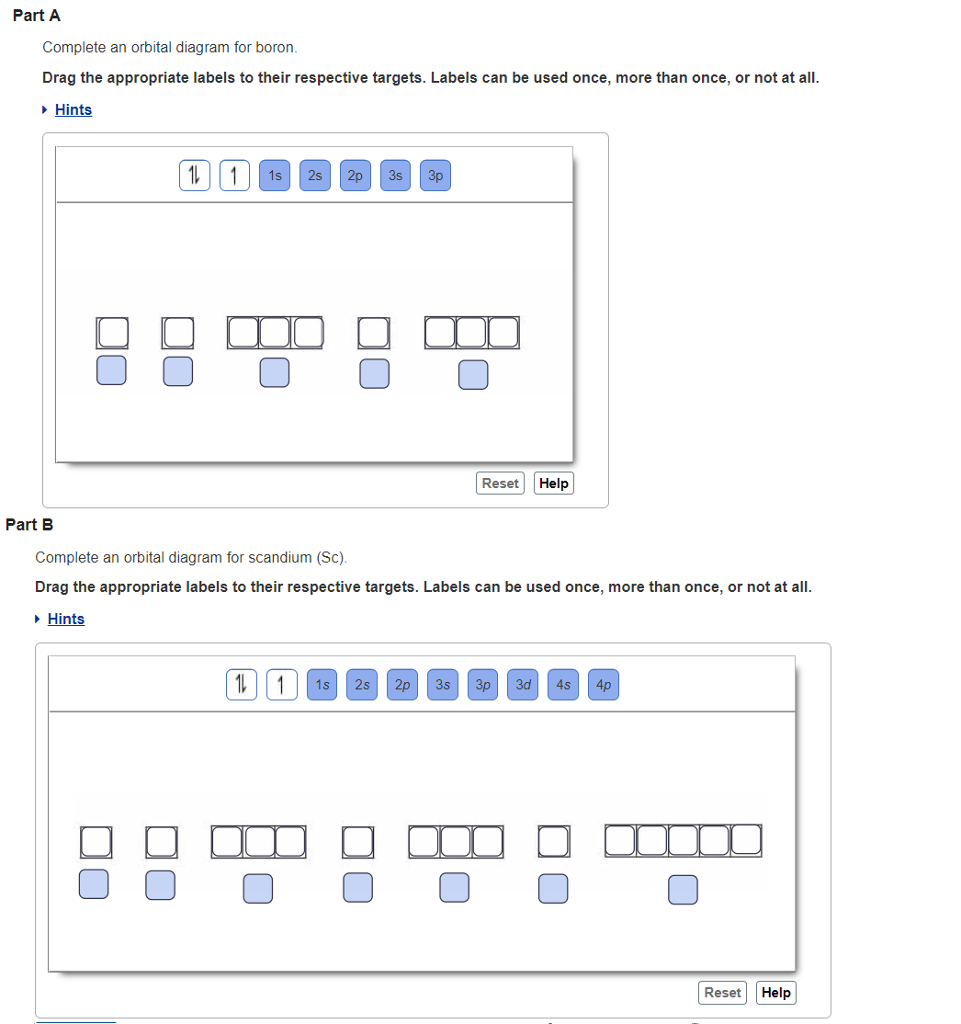
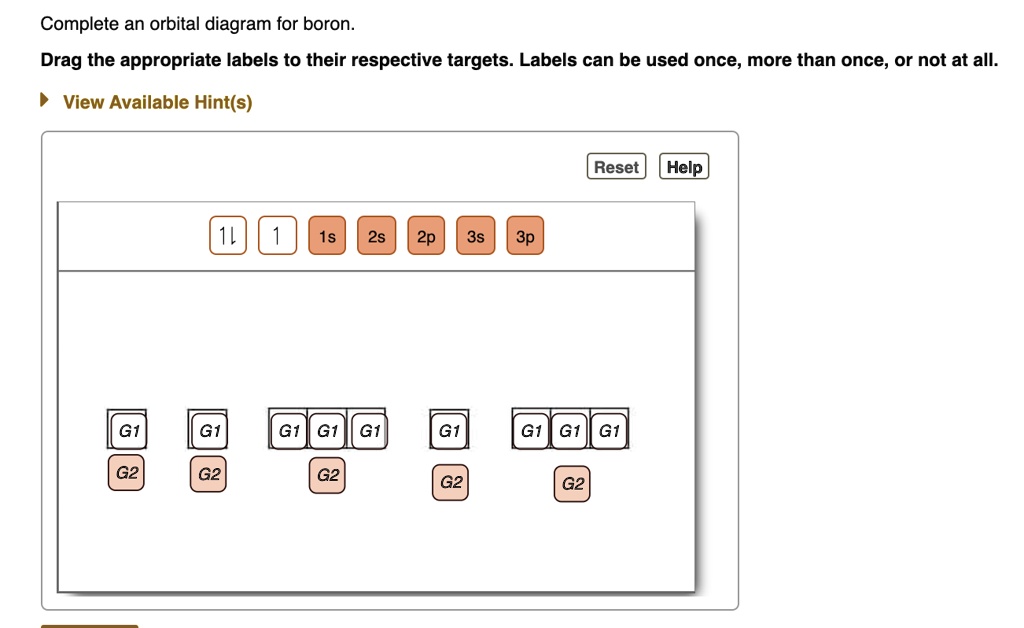
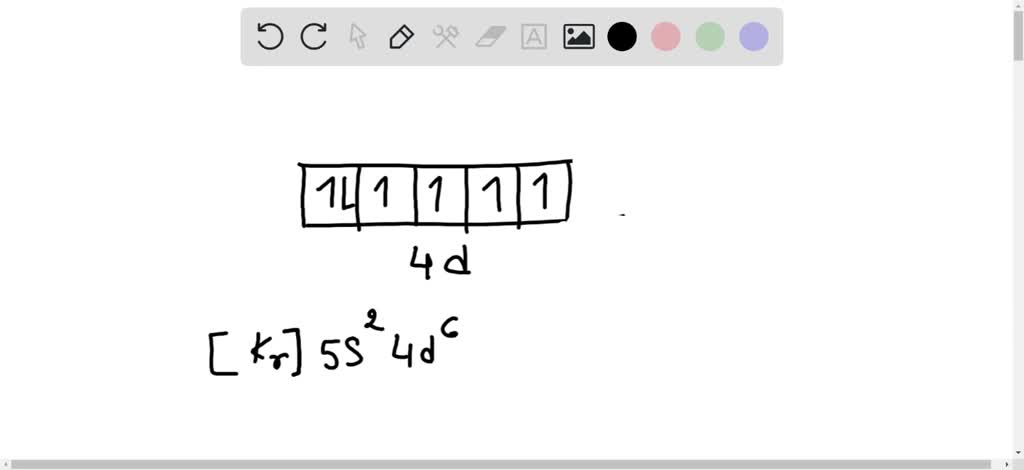
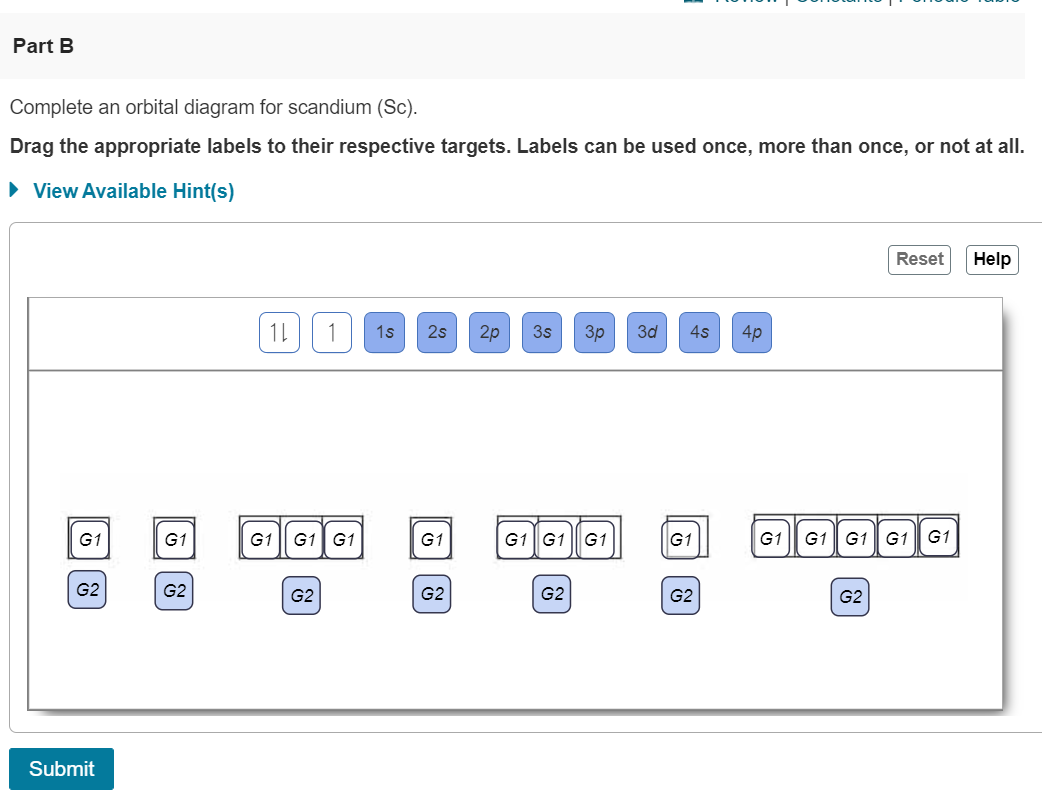
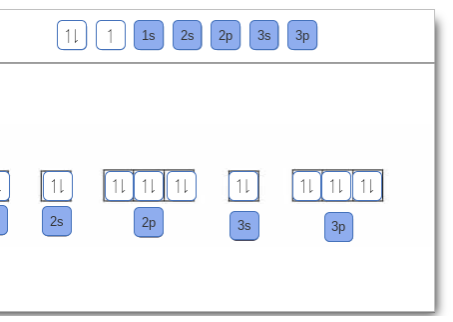
Orbital filling diagram for boron. The number of unpaired electrons in an atom can be determined from the orbital filling diagrams and correctly applying Hund's rule. Boron has one unpaired electron, while carbon has two, and nitrogen has three. How many unpaired electrons does fluorine have? 1. 3.2.4 State the maximum number of orbitals and electrons found in each sub-level. 3.2.5 Apply the Aufbau principle, Hund's rule and Pauli exclusion principle to construct electron configurations (extended and noble gas format) and orbital diagrams to show the locations of electrons for atoms up to Z = 18. 3.2.6 Define valence electrons. What is the electron configuration, abbreviated configuration and orbital filling diagram for the element Boron? Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of; Question: Draw an orbital diagram for boron. Use this tool to draw the orbital diagram. Draw an orbital diagram for scandium (Sc). Use this tool to draw the orbital diagram. How many orbitals are there in the third shell (n = 3)? Express your answer numerically ...
1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium The next element is boron with 5 electrons. The orbital diagram for boron as shown has the one electron in the 2p orbital. The electron can be placed in any of the three 2p orbitals. The electron configuration for boron is 1s 2 2s 2 2p 1. Boron (B) electron configuration with full orbital diagram Boron is the 5th element in the periodic table and the first element in group-13. The atomic number of boron is 5 and its symbol is 'B'. The standard atomic mass of boron is 10.806. The period of boron is 2 and it is a p-block element. The first transition series represents the filling of what orbital? the 3d orbital How many dots would a Lewis dot diagram for Boron have? The Lewis dot diagram for Boron has three dots. What is...
If you want to learn how to draw orbital filling diagrams, you need to follow The electron configuration of boron is 1s²2s²2p¹, which means that. Boron is the fifth element with a total of 5 electrons. In writing the electron configuration for Boron the first two electrons will go in the 1s orbital. Problem: Part A. Complete an orbital diagram for boron.Draw orbital diagrams, and use them to derive electron configurationsTo understand how to draw orbital diagrams, and how they are used to write electron configurations.The electron configuration of an element is the arrangment of its electrons in their atomic orbitals. Electron configurations can be used to predict most of the chemical ... The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ...
The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of. The next element is beryllium which has four electrons. The orbital diagram for beryllium is shown here.
1. Orbital Filling Diagram 02 Ex. 2, Electron Configuration 02 Ex. (gives the most information) Is (quicker to draw than orbital filling diagrams) Dot Pb 3. Electron Dot shows only the valence (outer energy level) electrons Oxygen atom Ex. 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following ...
Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium Where are the Electrons? Write the full electron configuration, short-hand electron configuration, and fill in the orbital diagrams, for the following elements. 1.
The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of: This problem has been solved!
An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. Show transcribed image text Draw an orbital diagram for boron.
Boron is the fifth element with a total of 5 electrons. In writing the electron configuration for Boron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for B goes in the 2s orbital. The remaining electron will go in the 2p orbital.
The number of unpaired electrons in an atom can be determined from the orbital filling diagrams and correctly applying Hund's rule. Boron has one unpaired electron, while carbon has two, and nitrogen has three.









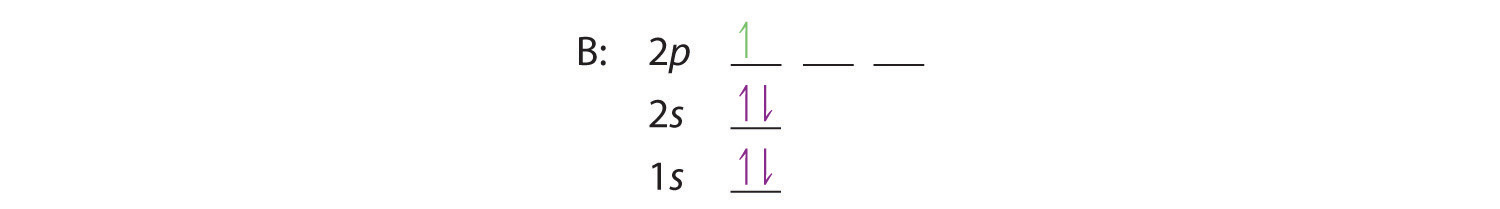












0 Response to "39 orbital filling diagram for boron"
Post a Comment