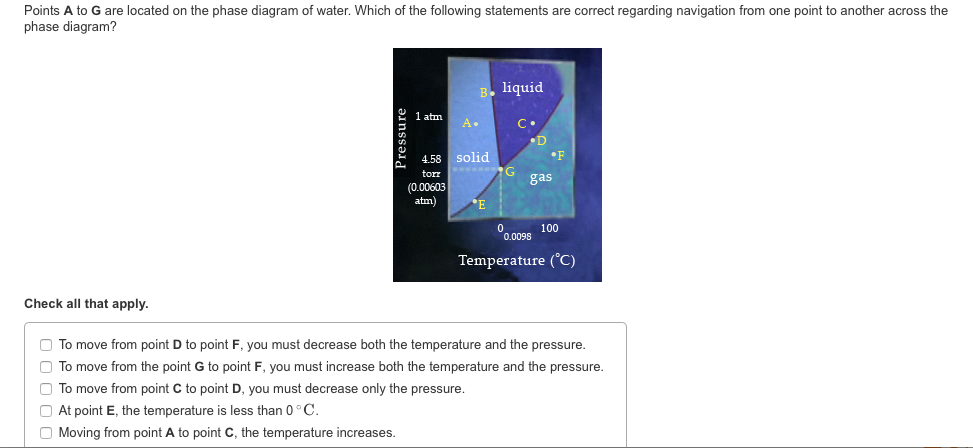
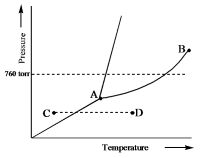
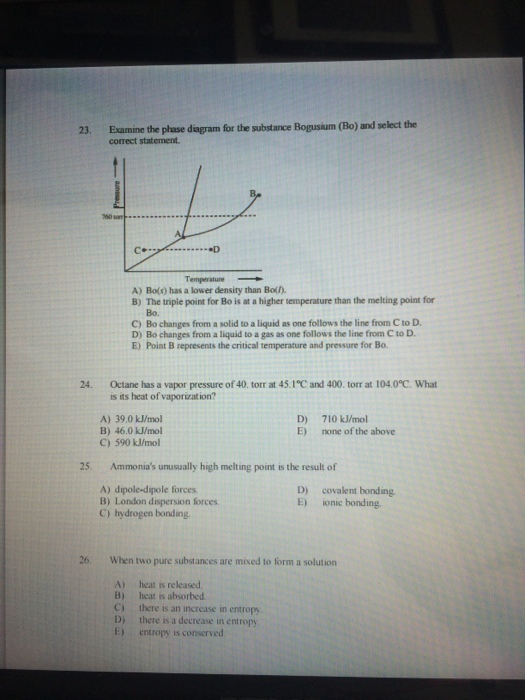
39 examine the phase diagram for the substance bogusium (bo) and select the correct statement.
Transcribed image text: Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement Pressure 760 ton AL C. •D Temperature O Point B represents the triple point for Bo. Bo changes from a solid to a gas as one follows the line from C to D. O Bo changes from a solid to a liquid as one follows the line from C to D. Bo(s) has a lower density than Bol). Solution for Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. B. 760 torr A •D Temperature A. Bo(s) has a lower…
'Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement L 760 tont Temperatue Bo(s) has a lower density than Bol).4 answers · Top answer: Okay. So, we've sketched out a phase diagram here. We started with the liquid vapor pressure ...

Examine the phase diagram for the substance bogusium (bo) and select the correct statement.
Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. ... pressure in a mixture of un-reacting gases is equal to the sum of the partial pressures of the individual gases"is a statement of....Law. Dalton's Law Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. A. Bo(s) has a lower density than Bo(l). B. ... Select the pair of substances in which the one with the higher vapor pressure at a given temperature is listed first. A. 3. Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. (a) Bo(s) has a lower density than Bo(l). (b) The triple point for Bo is at a higher temperature than the melting point for Bo. (c) Bo changes from a solid to a liquid as one follows the line from C to D. (d) Bo changes from a liquid to a gas as one follows the line from C to D. (e) Point B ...
Examine the phase diagram for the substance bogusium (bo) and select the correct statement.. Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. A) Bo(s) has a lower density than Bo(l) B) the triple point for Bo is at a higher temperature than the melting point for Bo C) Bo changes from a solid to a liquid as one follows the line from C to D Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. 11ea8ef8_2b5c_3c2d_ab0a_fb385d63c085_TB5833_00 A) Bo(s) has a lower density than Bo(l). B) The triple point for Bo is at a higher temperature than the melting point for Bo. C) Bo changes from a solid to a liquid as one follows the line from C to D. D) Bo changes from a liquid to a gas as one follows the ... C bo changes from a solid to a liquid as one follows the line from c to d. 9 examine the phase diagram for the substance bogusium bo and select the correct statement. A 224 l high pressure reaction vessel is charged with 03910 mol of iron powder and 12 atm of oxygen gas at standard temperature. Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. low temperatures and high pressures. Deviations from the ideal gas law are greater at
Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. a. Bo(s) has a lower density than Bo(l). b. The triple point for Bo is at a higher temperature than the melting point for Bo. c. Bo changes from a solid to a liquid as one follows the line from C to D. d. Bo changes from a liquid to a gas as one follows the line from C to D. e. Point B represents the critical temperature and pressure for Bo. Examine the phase diagram for the sibstance Bogusium (Bo) and select the correst statement. A) Bo(s) has a lower density than Bo(l). B) The triple point for ... Examine the phase diagram for the substance bogusium bo and select the correct statement. B the triple point for bo is at a higher temperature than the melting point for bo. 2 Component Phase Diagrams Examine the following phase diagram and identify the feature represented by point a. Oct 11, 2012 · Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. SEE QUESTION 10 Image A) Bo(s) has a lower density than Bo(l). B) The triple point for Bo is at a higher temperature than the melting point for Bo. C) Bo changes from a solid to a liquid as one follows the line from C to D. D) Bo changes from a liquid to a gas as one follows the line from C to D.
Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. 24. Octane has a vapor pressure of 40. Torr at 45.1 Degree C and 400. Torr at 104 .0 Degree C . What is its heat of vaporization? 25. Ammonia?s unusually high melting point is the result of 26. When two pure substance are mixed to form a solution Question: Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement Preure 760 C. .D Temperature Multiple Choice The Henry's Law constant (K) for carbon monoxide in water at 25°C is 9.71 x 10-4 mol/ (L-atm). How many grams of CO will dissolve in 1.00 L of water if the partial pressure of CO is 5.25 atm? Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. Pressure В. 760 torr A C- -D Temperature A) Bo (s) has a lower density than Bo (l). B) The triple point for Bo is at a higher temperature than the melting point for Bo C) Point B represents the critical temperature and pressure for Bo. Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. askedSep 3, 2019in Chemistryby NyanCat. A. Bo changes from a liquid to a gas as one follows the line from C to D. B. Bo(s) has a lower density than Bo(l). C. Point B represents the critical temperature and pressure for Bo. D.
23. Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. 24. Octane has a vapor pressure of 40.
Question: 4726.97 - [51.45 + 14367.8) 7) Examine the phase diagram for the yet unknown substance Bogusium (Bo) and select the correct statement. Re: 68.9 ...
3. Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. (a) Bo(s) has a lower density than Bo(l). (b) The triple point for Bo is at a higher temperature than the melting point for Bo. (c) Bo changes from a solid to a liquid as one follows the line from C to D. (d) Bo changes from a liquid to a gas as one follows the line from C to D. (e) Point B ...
Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. A. Bo(s) has a lower density than Bo(l). B. ... Select the pair of substances in which the one with the higher vapor pressure at a given temperature is listed first. A.
Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. ... pressure in a mixture of un-reacting gases is equal to the sum of the partial pressures of the individual gases"is a statement of....Law. Dalton's Law






















0 Response to "39 examine the phase diagram for the substance bogusium (bo) and select the correct statement."
Post a Comment