41 lewis dot diagram for pcl3
Nitrogen trichloride (NCl3) lewis dot structure, molecular geometry, polar or non-polar, hybridization. Nitrogen trichloride is a very explosive substance that appears like an oily liquid with the chemical formula NCl3. It smells similar to chlorine. It has a dipole moment of 0.6 D that shows it is moderately polar.
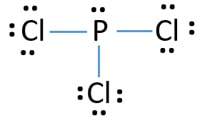
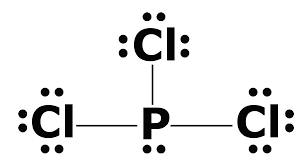
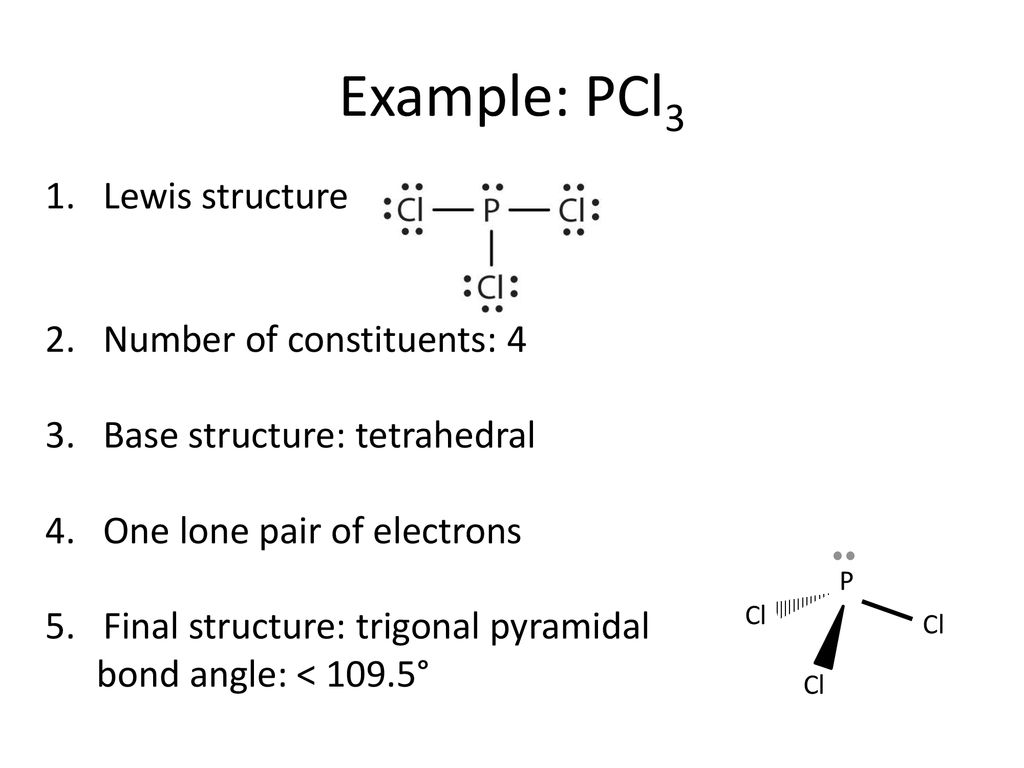
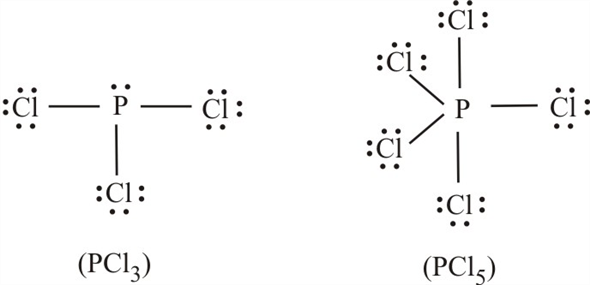
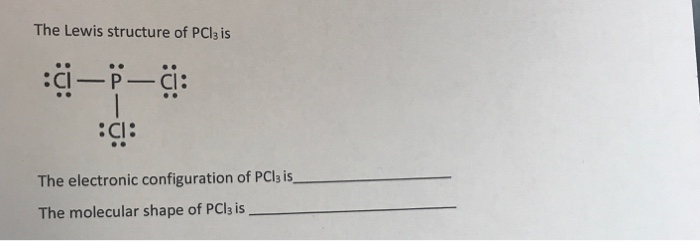
We can clearly see from the lewis diagram that in PCl3, phosphorus is forming three sigma bonds with 3 chlorine atoms. With that 2 lone pairs are present on the phosphorus atom. This concept very well explains the hybridization of PCl3 which is sp3. Another simple formula can also give us the hybridization of PCl3.
Lewis dot diagram for pcl3. Pcl5 is a lewis acid. The lewis structure or lewis dot diagram. Lets do the lewis structure for pcl3. Find out a to z information of pcl3 ie phosphorus trichloride here. Since they are in the same group on the periodic table they each have the same number of electrons 7 their structures are similar.
Lewis dot diagram for pcl3
Thanks. Draw a Lewis Dot structures for the ionic compounds: KF CaBr2 Draw a Lewis Dot structures for the covalent compounds: OF2 PCl3 CH4 H2 Thank you very much. Basic chemistry- Lewis Dot structures. Test Preparation. SAT Subject Test Preparation. BCeagles January 8, 2006, 5:12pm #1 <p>Hey, could some one please help me with these. ...
I quickly take you through how to draw the Lewis Structure of PCl3, phosphorous trichloride. I also go over hybridization, shape and bond angle.
• (3c) ·Lewis dot diagrams are used to represent valence electrons in an element. Structural formulas show the arrangements of atoms and bonds in a molecule and are represented by Lewis dot structures. • Draw Lewis dot diagrams to represent valence electrons in elements and draw Lewis dot structures to show covalent bonding.
Lewis dot diagram for pcl3.
We can clearly see from the lewis diagram that in PCl3, phosphorus is forming three sigma bonds with 3 chlorine atoms. With that 2 lone pairs are present on the phosphorus atom. This concept very well explains the hybridization of PCl3 which is sp3. How is the phosphorus trichloride ( PCl3 ) made?
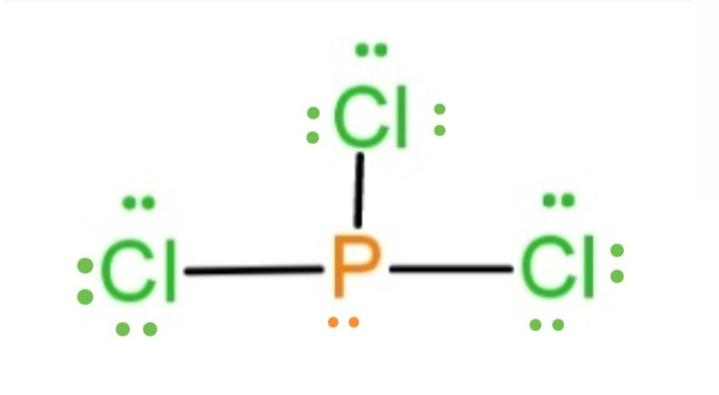
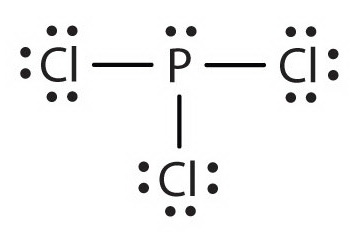
PCl 3 (Phosphorus Trichloride) Lewis Structure. Phosphorus trichloride (PCl 3) contains three chlorine atoms and one phosphorus atoms. In PCl 3 lewis structure, each chlorine atom is joint with center phosphorus atom through a single bond. Also, there is a lone pair on phosphorus atom. In this tutorial, we will learn how to draw the lewis structure of PCl 3 with all theories.
The structure of pcl3 is a phosphorus with lone pair two electrons and 3 chlorine atoms attached by single bond where each has pairs lewis symbols numbers on the staar reference chart 1a 2a 3a etc match number of valence electrons as shown table with electron dot diagrams phosphorus trichloride is a chemical compound of and chlorine having the ...
Lewis Electron-dot Diagram For Pcl3. October 09, 2021. Http Www Youtube Com Watch V Qojoaosk5ui Lewis Chemistry Math Equations. Pin On Chemistry What Is Chemistry. Pcl3 Lewis Structure Phosphorus Trichloride In 2021 Math Equations Lewis Chemical Formula.
Lewis dot structure. 9. In the Lewis formula for sulfur dioxide, SO2, the number of lone pairs of electrons around the sulfur atom is. 1. ... PCl3? ClO^3-17. The correct Lewis structure for Cl2CO is (the 2 Cl's and the O are bound to the C and not to each other): 18. Which of the following is the Lewis electron dot structure for carbon monoxide ...
What is the Lewis dot structure for pcl3? PCl3 (Phosphorus Trichloride) Lewis Structure. Phosphorus trichloride (PCl3) has 3 chlorine atoms as well as one phosphorus atoms. In PCl3 lewis structure, each chlorine atom is joint with facility phosphorus atom with a solitary bond. Likewise, there is an only set on phosphorus atom.
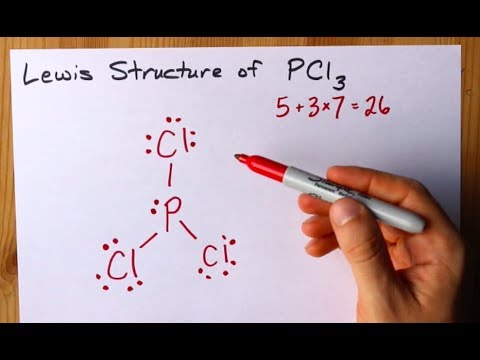
Drawing the Lewis Structure for PCl 3. Viewing Notes: PCl 3 is similar to PBr 3 and PF 3.If you can do those Lewis structures PCl 5 will be easy.; In the PCl 3 Lewis structure Phosphorus (P) is the least electronegative so it goes in the center.; In the Lewis structure for PCl 3 there are a total of 26 valence electrons. hree pairs will be used in the chemical bonds between the P and Cl.
9. Draw a Lewis dot diagram for the barium atom. 10. Draw the Lewis dot diagram for the silicon atom. I I, Draw the Lewis dot diagram for the iodine atom. 12. Draw the Lewis dot diagram for the xenon atom. 13. Hypothesize: Why are noble gases considered to be non-reactive? Your group will check your answers with the instructor before moving on.
1 point is earned for a correct Lewis diagram. (ii) In Box Y below, draw the complete Lewis electron-dot diagram for the other compound, which is a structural isomer of the compound represented in Box X. Include any lone (nonbonding) pairs of electrons. 1 point is earned for a correct Lewis diagram.
What is the Lewis dot structure for pcl3? PCl3 lewis structure In this lewis structure of PCl3, center phosphorus atom has made three single bonds with three chlorine atoms. There is a lone pair on center phosphorus atom and each chlorine atom also has three lone pairs. Also, there are no charges on atoms in PCl3 lewis structure.
The Lewis Dot Structure for PCl 3:. The Lewis dot structure for any molecule can be found by following a general set of rules consisting of 5, or sometimes 6 steps.
Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. It is a toxic compound but is used in several industries.
The PCl 3 Lewis structure has the typical case of phosphorus P in the center with 3 bonds to 3 other atoms. Phosphorus is from the same column as nitrogen in the periodic table, meaning that P and N generally have the same bonding structure. Note the lone pair (dots without bonds) on top of P, just like for N in the previous example for NH 3 .
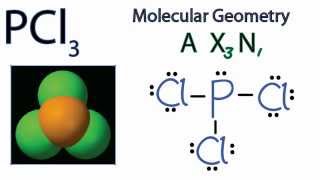
Answer: A quick Google search will help you find it. If you want to know how this is found, check How to Draw A Lewis Structure In case you were wondering, the molecular geometry is trigonal pyramid.
PCL3 Lewis Structure. In the Lewis structure of PCL3, there are two chemical compounds.One is phosphorous (P), and the second is chlorine (Cl). To draw the PCL3 lewis structure, follow the below instructions. First of all, find out the total number of valence electrons in the PCL3 using the periodic table.
For structure, calculate total number valence electrons t. How to draw lewis structure for pcl3. Looking at lewis structure we can see that th. This is a video worked example for the lewis structure and molecular geometry of phosphorus trichloride (pcl3). A stepbystep explanation of how to draw the pcl3 lewis structure (phosphorus trichloride).
Every element in the second group (column) has 2 dots in the electron dot structure (also known as the lewis dot structure): Beryllium, Magnesium, Calcium, Strontium, Barium, and Radium.
The lewis dot structure for pcl3. Consider initial downwind evacuation for at least 300 meters 1000 feet. Write the symbol and fill in the Lewis Dot Diagrams for the 1st four rows and all elements in groups columns 1 2 and 1318. It is a toxic compound but is used in several industries. Write the Lewis dot symbol for. A N2O B CS2 C PH3 D CCl4 E NO2.
The molecular geometry of PCl3 is trigonal pyramidal and its VSEPR notation is AX3E. The "A" represents the central atom (the phosphorus), each X represents a chlorine atom, and the E represents the lone pair. You can watch me draw the Lewis Dot Diagram for PCl3 here: How to Draw the Lewis Structure of PCl3 (phosphorus trichloride) Watch later
5. Draw the dot structure for the following covalent compounds. State the molecular shape or structure (linear, bent, trigonal planar, trigonal pyramidal, or tetrahedral). H2O CH4 CO2 SO3. NF3 F2 N2. 6. Draw the dot structure for the following ionic compounds: NaBr Al2O3 CaBr2 MgO. 7.
A step-by-step explanation of how to draw the PCl3 Lewis Dot Structure (Phosphorus Trichloride).For the PCl3 structure use the periodic table to find the tot...
Now lets draw the Lewis dot structure for PCl 5 phosphorous pentachloride. Vsepr theory bond angles NSF3 SiF4 POF3. Pcl5 Phosphorous Pent...
Answer: Count total valence electrons. Chlorine 3x7=21; Phosphorus 1+5=5; 21+5=26. This tells us that these are all the electrons we have available to use in our structure. Next we write down P as the central atom and connect a single bond to each Cl. Each bond counts as two electrons so now we h...

Warmup fill out the table below. make sure to draw the lewis structure in pencil! try your best! molecule so3 pcl3 o3 sicl4 beh2 total valence electrons.






















0 Response to "41 lewis dot diagram for pcl3"
Post a Comment