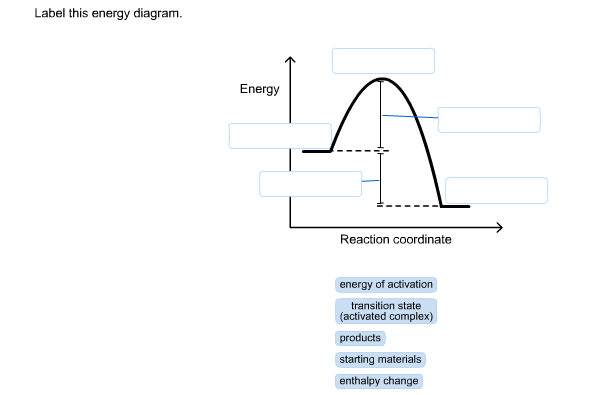
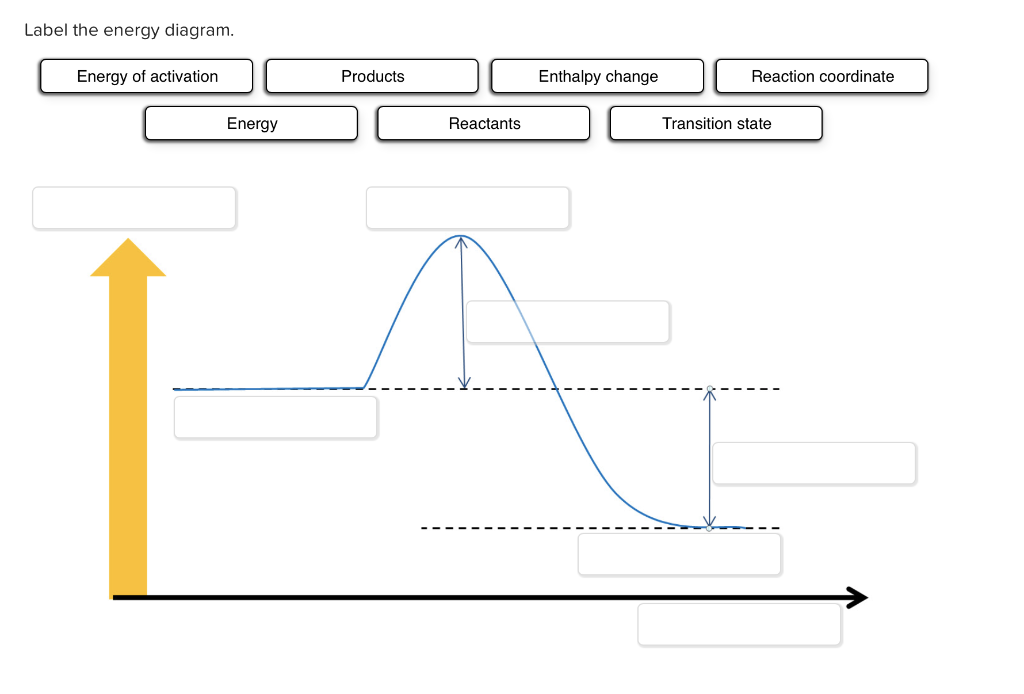
40 label this energy diagram
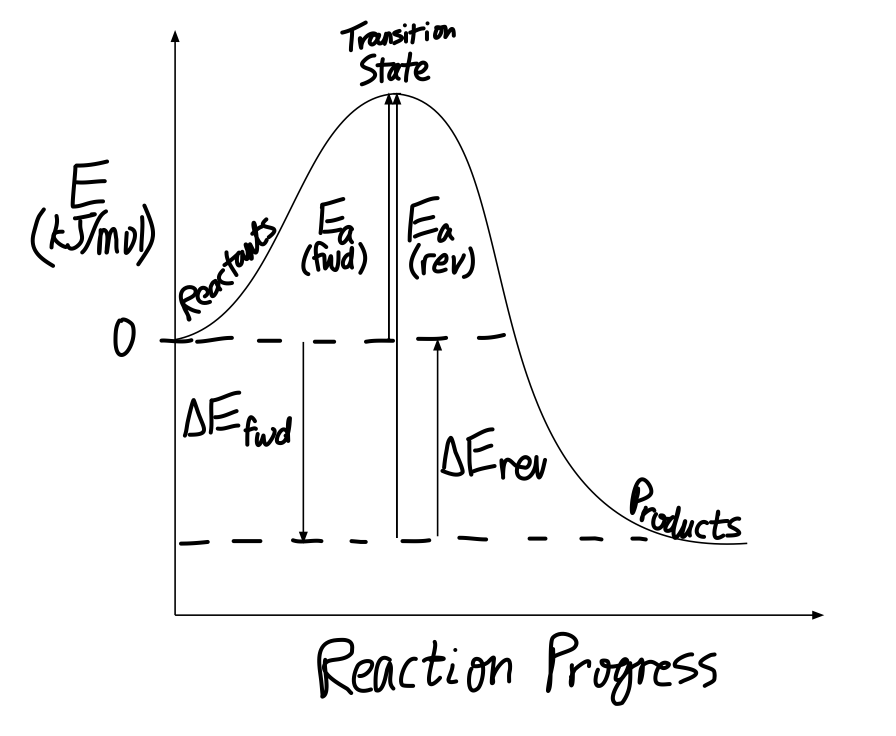
Representing a Reaction with a Potential Energy Diagram (Student textbook page 371) 11. Complete the following potential energy diagram by adding the following labels: an appropriate label for the x-axis and y-axis, E a(fwd), E a(rev), ΔH r. a. Is the forward reaction endothermic or exothermic? b.
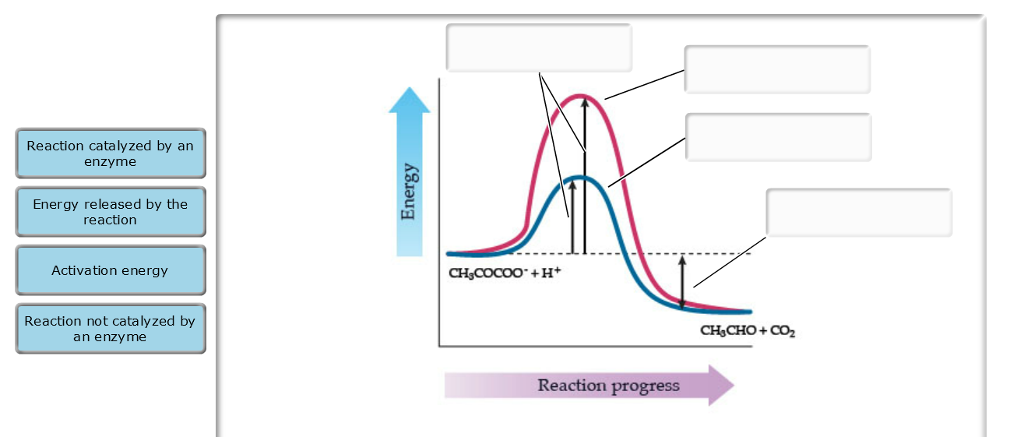
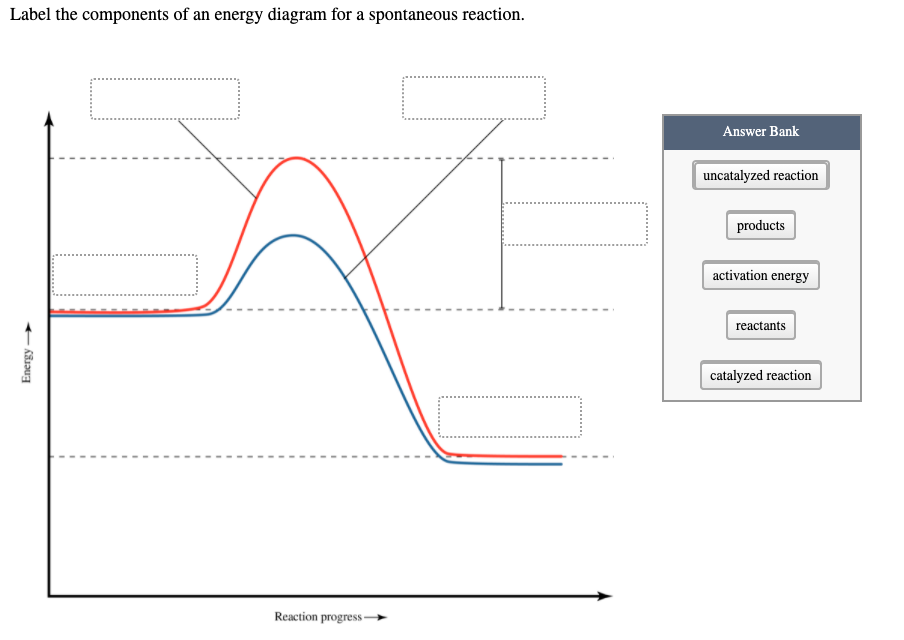
Label the energy diagram and answer the question that follows%(1). Catalyzed reactions have a lower activation energy (rate-limiting free energy of activation) than the corresponding uncatalyzed reaction, resulting in a higher reaction rate at the same temperature and for the same reactant concentrations.
1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.

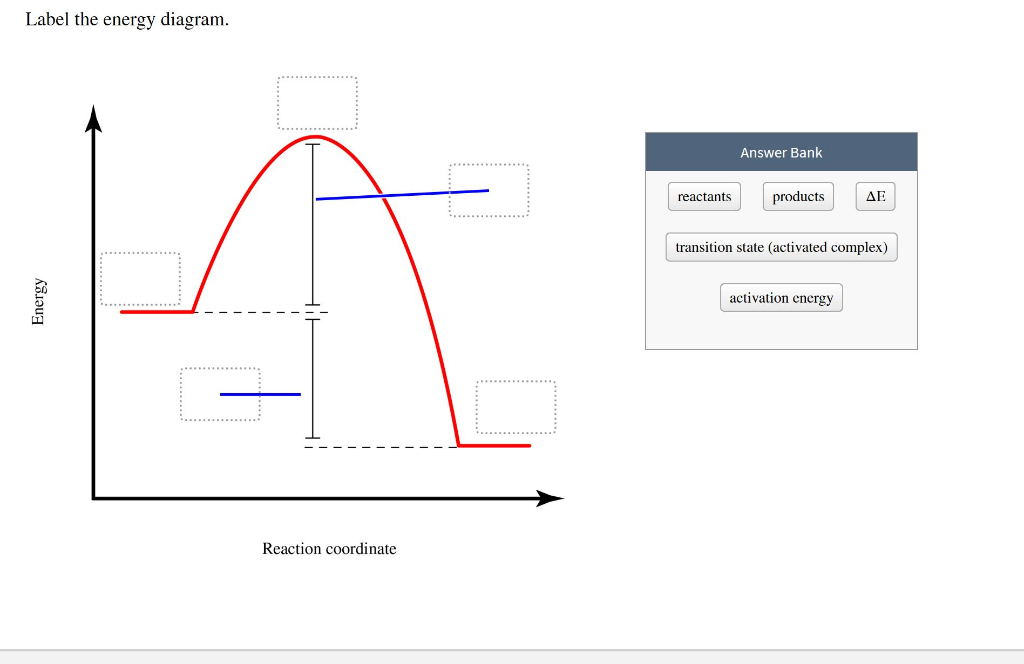
Label this energy diagram
Potential energy diagrams Consider an arbitrary potential energy shown schematically below. There are a number of important qualitative features of the behavior of the system that can be determined by just knowing this curve. The first thing to notice is that since the kinetic energy
Nutrient cycling and energy flow in ecosystems can you label this diagram showing how nutrients and energy flow in an ecosystem. Dissipation of energy occurs as heat. The continuous intake of energy in photosynthesis replaces the energy dissipated to environment by respiration and biological activity and the system does not run down through the ...
Energy level diagrams and the hydrogen atom. It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states. If the electron in the atom makes a transition from ...
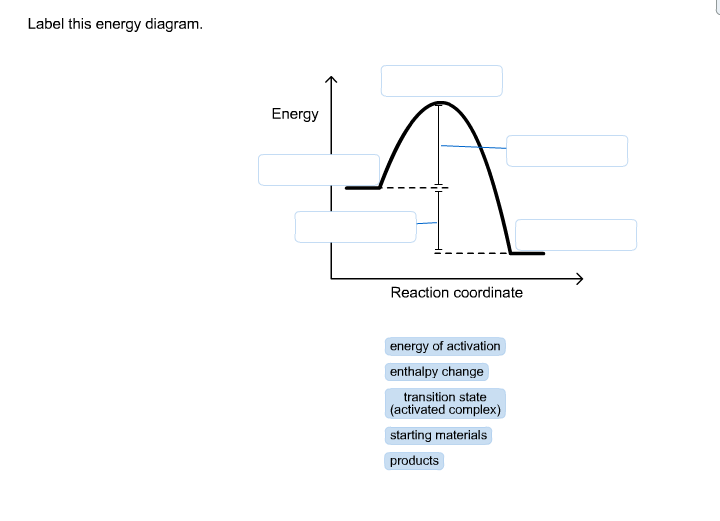
Label this energy diagram.
How would you draw and label energy diagrams that depict the following reactions, and determine all remaining values? Place the reactants at energy level zero . Chemistry Chemical Kinetics Potential Energy Diagrams. 1 Answer Truong-Son N. May 18, 2017
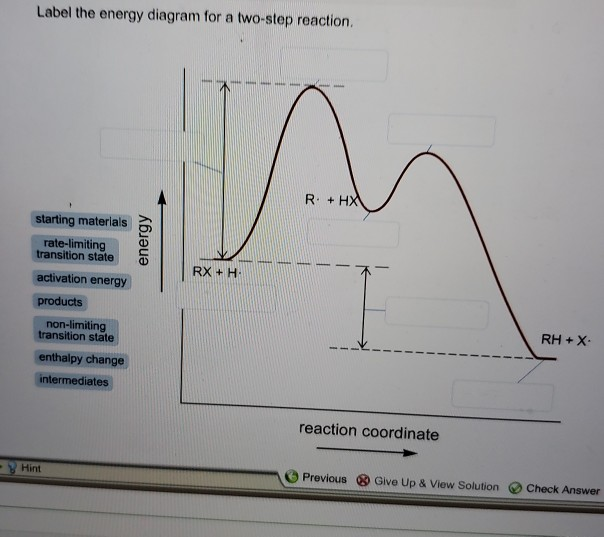
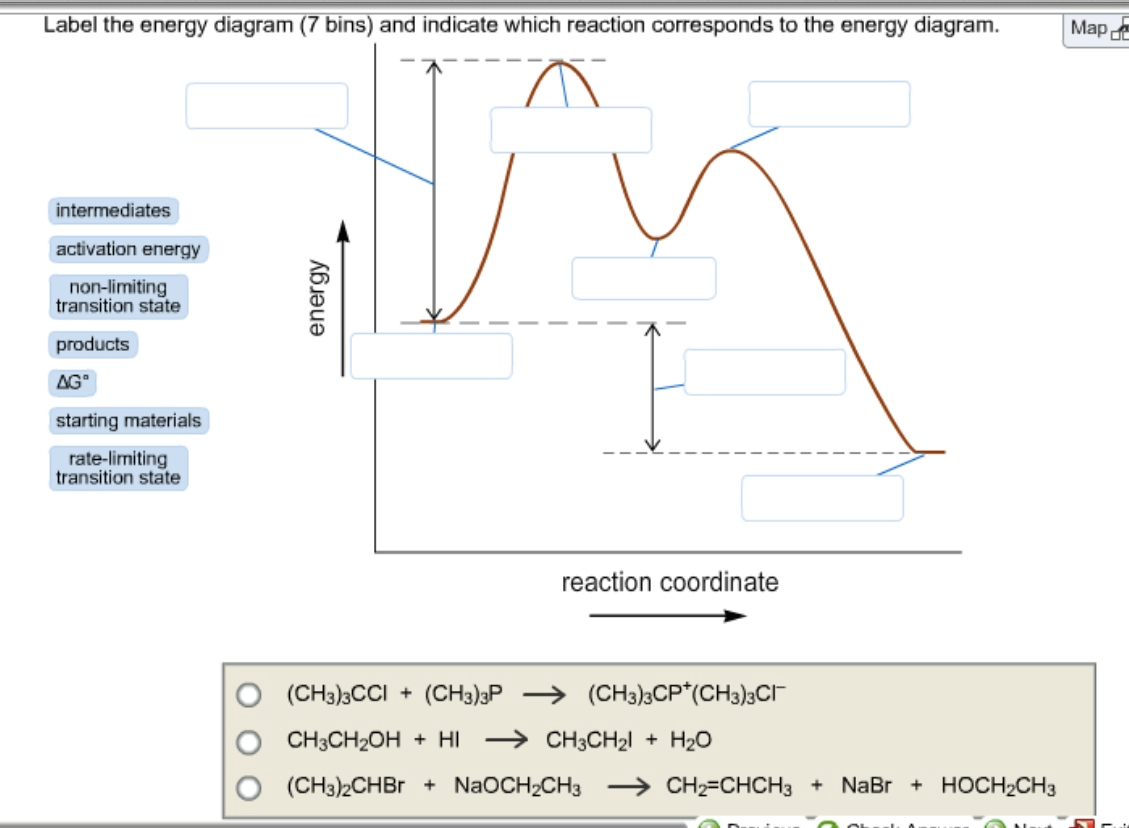
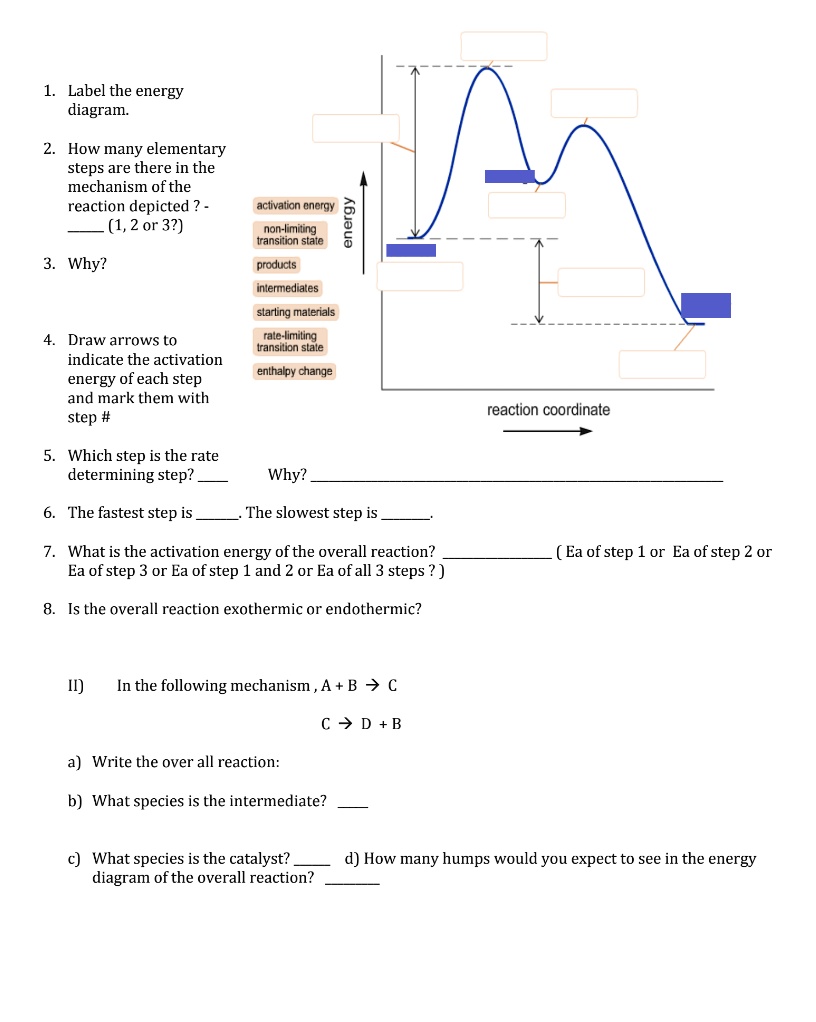
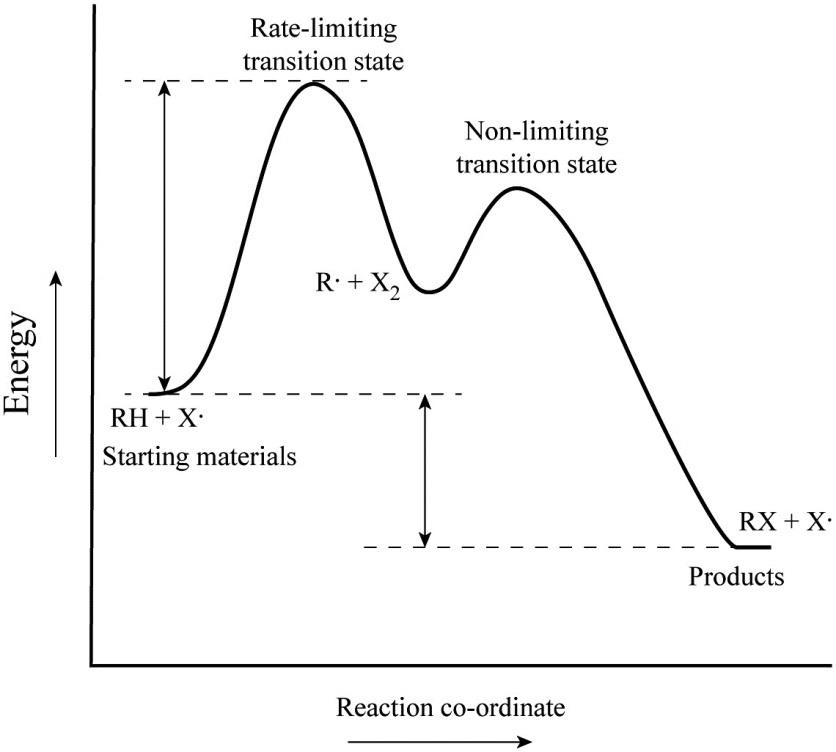
Label the energy diagram for a two-step reaction. Q. A reaction coordinate diagram is shown below for the reaction of A to form E. Answer the following questions.i) Identify the transition state (s)?ii) W... Q. Which reaction coordinate diagram represents a reaction in which the activation energy, Ea, is 50 kj.mol-1 and the ΔHrxn is -15 kj. mol-1?
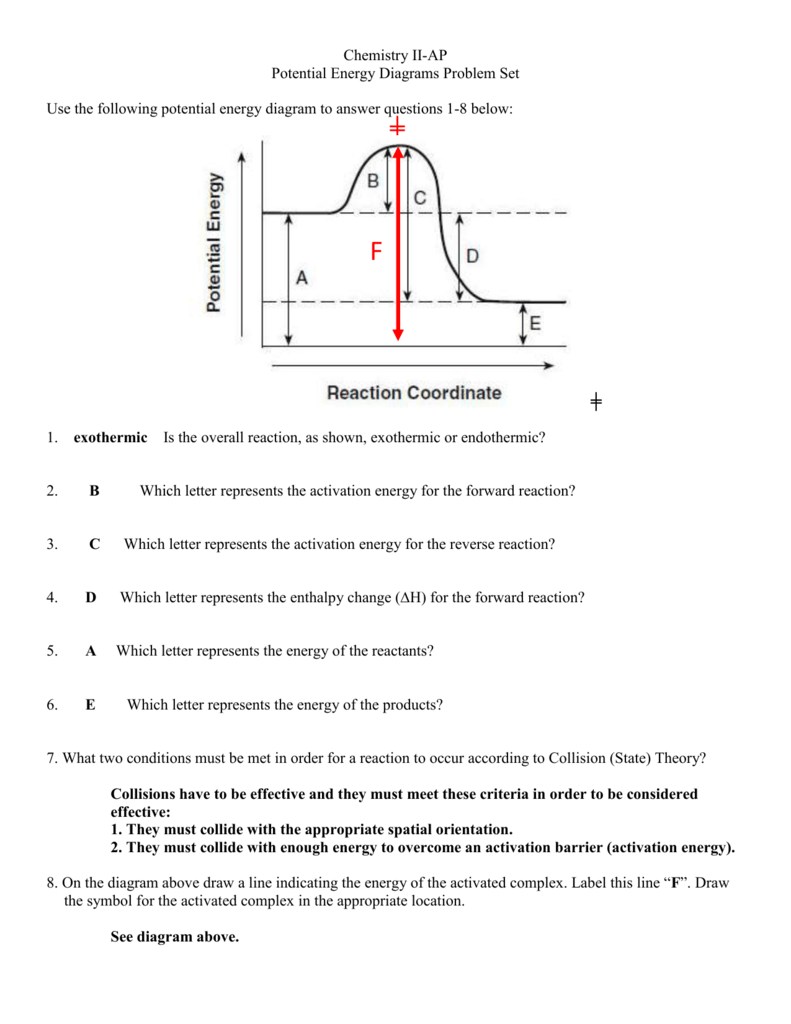
6. Draw an energy diagram for a reaction. Label the axis, PE of reactants = 350 KJ/mol, Ea = 100 KJ/mol, PE of products = 250 KJ/mol. 7. Is the reaction in # 6 exothermic or endothermic? Explain. Exothermic. The ΔH is -100 KJ/mol which means heat is released. 8. How could you lower the activation energy for the reaction in #6? Add a catalyst.
energy diagram . These diagrams can be useful in describing motion for all types of objects. Once you have the potential energy diagram for a situation, you can describe the motion of an object. The usual method of using potential energy diagrams is to: • start with the physical situation • use the physics to draw the potential energy diagram
The overall change in energy in a reaction is the difference between the energy of the reactants and products. Exothermic reactions The diagram shows a reaction profile for an exothermic reaction.
2. Draw and label two short horizontal lines to mark the energies of the reactants and products. 3. Draw the energy level diagram. There must be a hump in the curve to represent the energy level of the activated complex. 4. Draw and label the activation energy. Draw a horizontal line from the highest part of the curve towards the vertical axis.
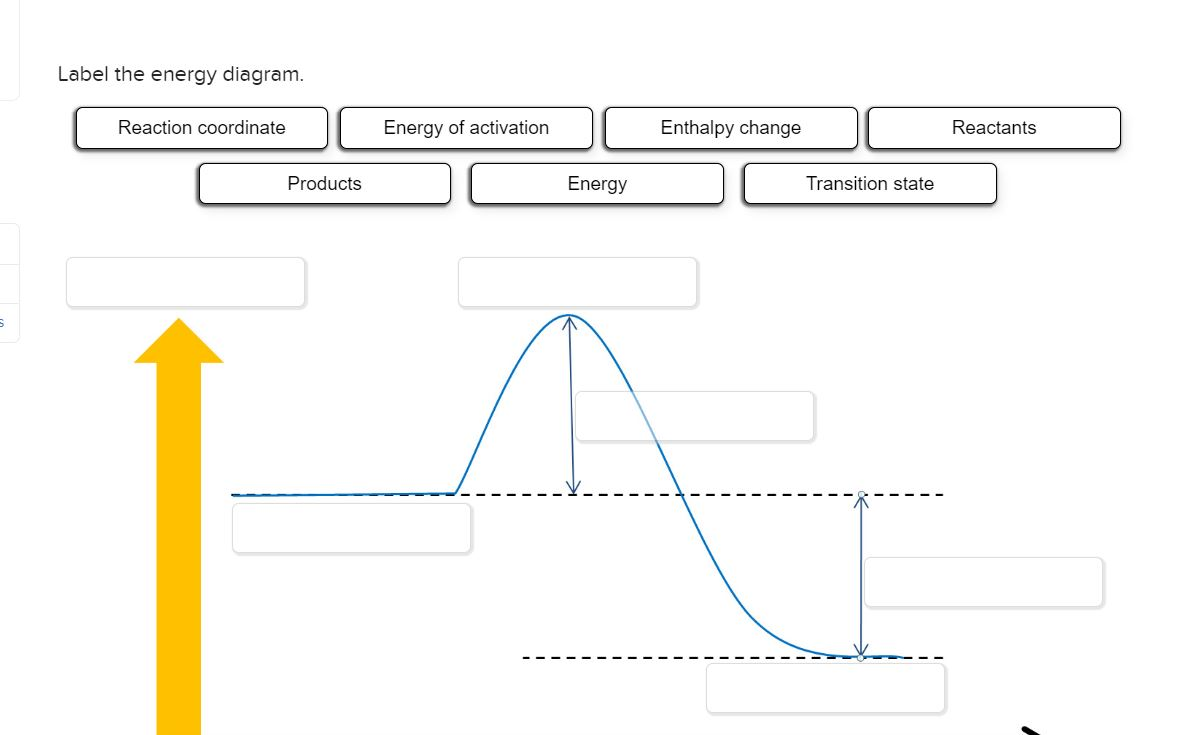
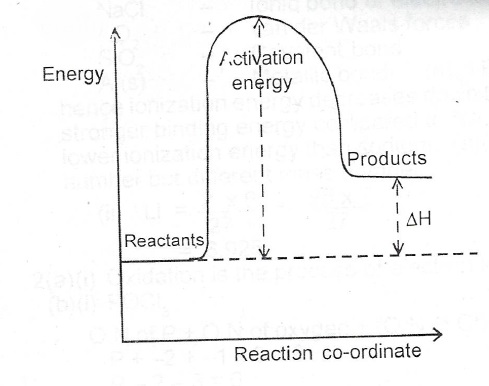
Problem: Label this diagram (energy of activations, transition state, products, starting materials, and enthalpy change) FREE Expert Solution. 89% (463 ratings) FREE Expert Solution. We're being asked to label the given energy diagram. Recall that an energy diagram is usually read from left to right.
Answer to Label the energy diagram (7 bins) and indicate which reaction corresponds to the energy diagram. The y-axis of the Maxwell-Boltzmann graph can be thought of as giving the number of molecules per unit speed. So, if the graph is higher in a given region. If playback doesn't begin shortly, try restarting your device.
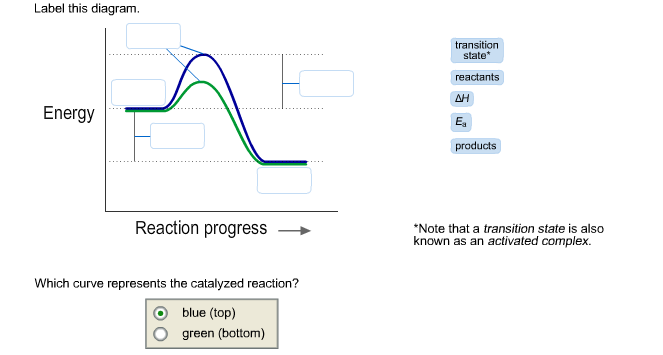
Show transcribed image text. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 98% (50 ratings) Transcribed image text: Label this diagram Which curve represents the catalyzed reaction? Blue (top) green (bottom)
This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
An Energy Profile is also referred to as an Energy Diagram or as a Potential Energy Diagram. An energy profile is a diagram representing the energy changes that take place during a chemical reaction. Enthalpy change, ΔH, is the amount of energy absorbed or released by a chemical reaction. On an energy profile, the enthalpy change for the ...
Recall that an energy diagram is usually read from left to right.. The components of a one-step energy diagram are: • Reactants: are placed on the left/beginning of the energy diagram • Products: are placed on the right/end of the energy diagram • Transition state: is the state with the highest energy in the energy diagram • Energy change (ΔE or ΔG˚): is the difference in energy ...
Below is a blank energy level diagram which helps you depict electrons for any specific atom. At energy level 2, there are both s and p orbitals. The 2s has lower energy when compared to 2p. The three dashes in 2p subshells represent the same energy. 4s has lower energy when compared to 3d. Therefore, the order of energy level is as follows: s ...
An enthalpy diagram allows us to easily see details of a chemical reaction. By knowing how to draw and label an enthalpy diagram we can see what the starting energy level is, how much energy is ...
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
a) Draw a diagram of the energy profile for this reaction. Label the diagram. b) State whether the reaction is endothermic or exothermic. c) Calculate the energy difference between the reactants and the products. d) Deduce the sign of the enthalpy change. e) Identify with a reason, which is more stable, the reactants of products. 8. (N04/S/2)
Draw an energy diagram for an endothermic reaction without a catalyst (use a solid line) and with a catalyst (use a dotted line). Label all parts of the diagram. Explain what a catalyst is and how a catalyst influences the rate of a reaction. Answer: A Catalyst lowers the activation energy so it increases the rate of reaction.
Label the energy diagrams below and complete the statements about each. reactants products released to stays the same Energy, E Energy, E decreases products reactants increases Efinal Einitial Efinal Einitial Einitial AE ΔΕ Jo Efinal Energy of system Energy of system absorbed from Energy is surroundings. Energy is _ surroundings.
Step-by-step discussion on the labels of the different areas in the potential energy diagram. In this examples, we are having an Endothermic type of reaction...
Frontiers · Label The Energy Diagram For A Two Step Reaction - Chemistry Archive October 28. potential energy diagrams ap chemistry a potential energy diagram plots the change in potential energy that occurs during a chemical reaction this first video takes you through all the basic.
Start studying Labeling an Energy Diagram. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
This photo about: Label This Energy Diagram, entitled as Label The Energy Diagram For A Two Step Reaction - Label The Energy Label This Energy Diagram - also describes Label The Energy Diagram For A Two Step Reaction - Label The Energy and labeled as: ], with resolution 3102px x 1093px
Label ΔH as positive or negative. Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases.























0 Response to "40 label this energy diagram"
Post a Comment