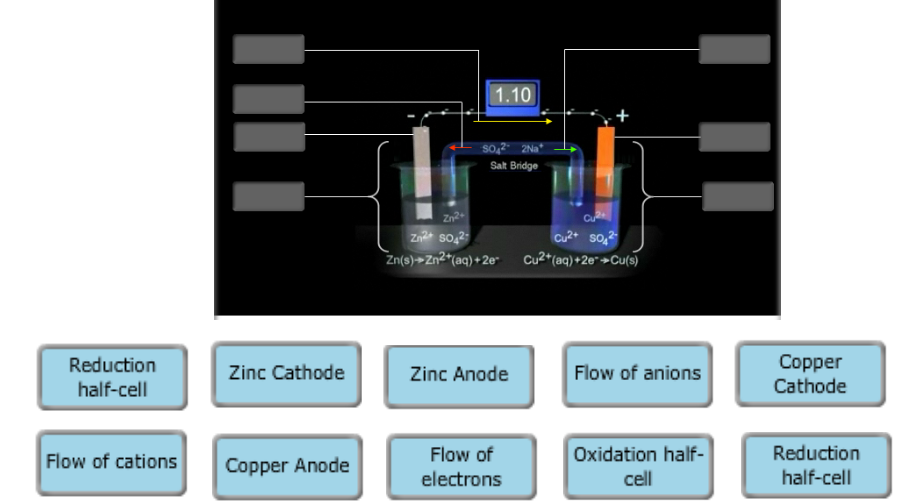
40 label the diagram according to the components and processes of a voltaic cell
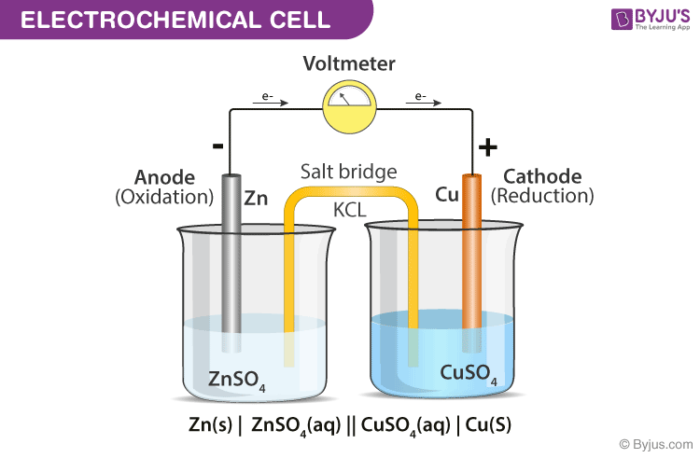
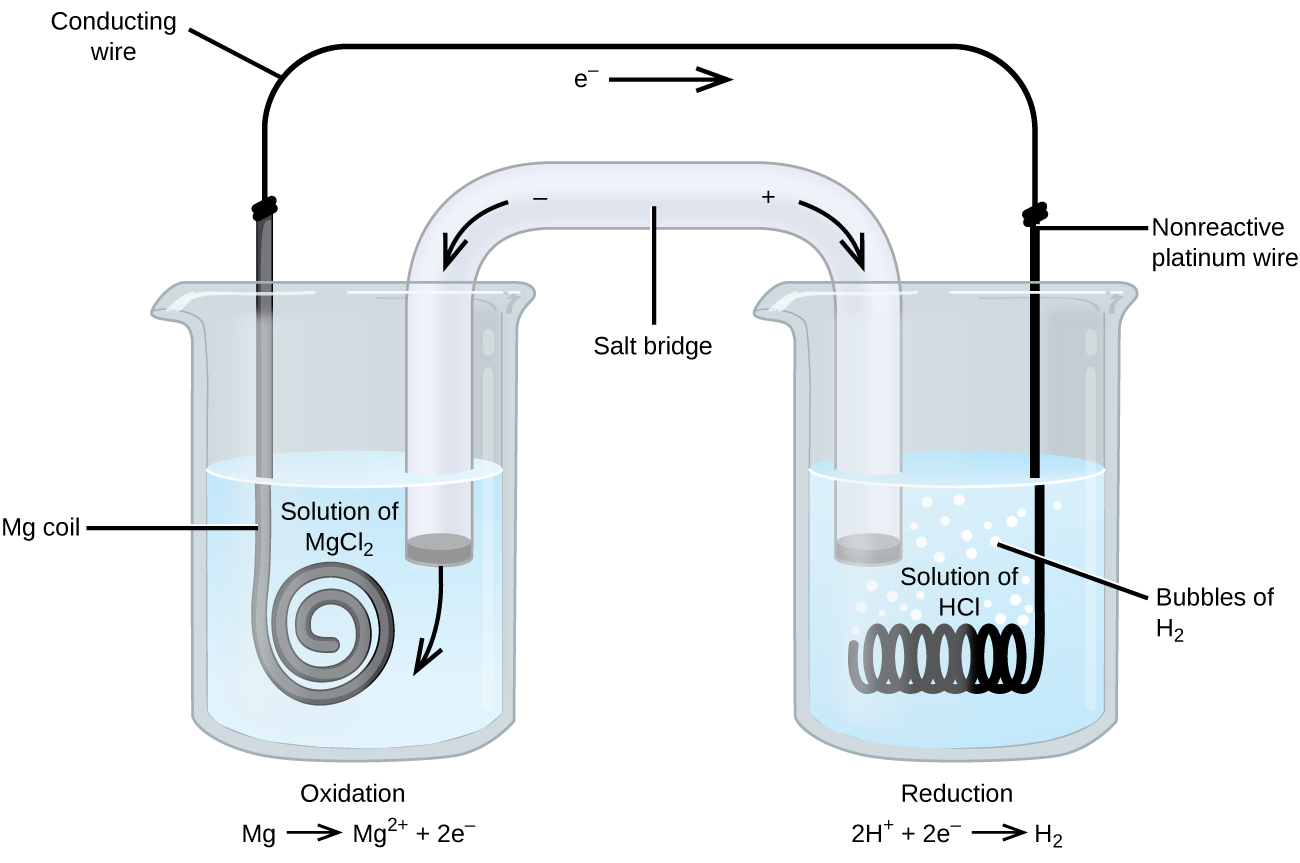
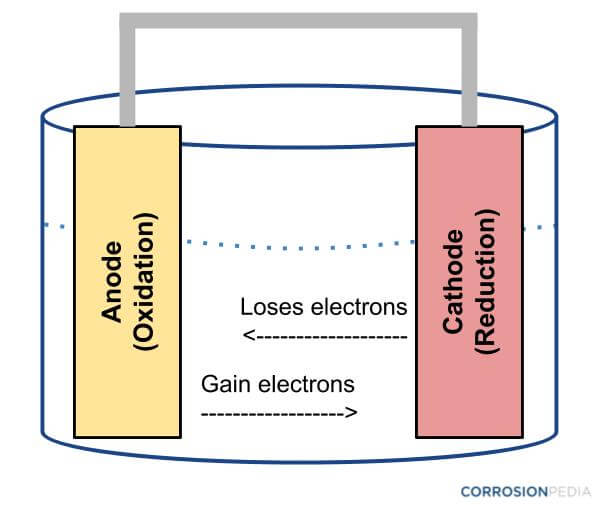
Goal: to describe the construction and operation of a voltaic cell Working Definitions. Electrical current is the movement of charged particles, either electrons or ions, through a conductor.. A voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. The important parts of a voltaic cell:. The anode is an electrode where oxidation occurs. Label the diagram according to the components and processes of a voltaic cell. Drag the appropriate labels to their respective targets. Question: Label the diagram according to the components and processes of a voltaic cell. Drag the appropriate labels to their respective targets.
Human Cell Diagram, Parts, Pictures, Structure and Functions The cell is the basic functional in a human meaning that it is a self-contained and fully operational living entity. Humans are multicellular organisms with various different types of cells that work together to sustain life.
Label the diagram according to the components and processes of a voltaic cell
A voltaic cell is made from magnesium and iron half-cells. Magnesium is a more reactive metal than iron. Which statement is correct when the cell produces electricity? A. Electrons are lost from Magnesium atoms. B. The concentration of Fe 2+ ions increases. C. Electrons flow from the iron half-cell to the Magnesium half-cell. Answer to label the diagram according to the components and processes of a voltaic cell. A voltaic cell has several components o o o o o o anode. Both types of cells use two electrodes that provide an electrical connection between systems that are separated in space. Block Diagram Of The Proposed Smart Rfid Label Download Electrolytic Cells. Voltaic cells use a spontaneous chemical reaction to drive an electric current through an external circuit. These cells are important because they are the basis for the batteries that fuel modern society. But they aren't the only kind of electrochemical cell. It is also possible to construct a cell that does work on a ...
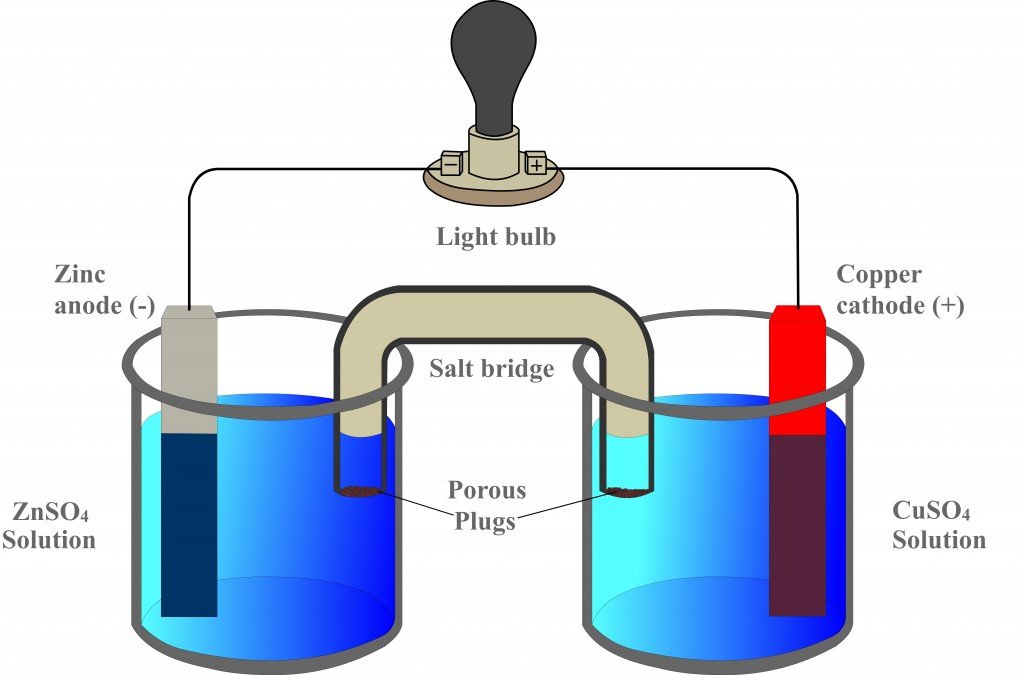
Label the diagram according to the components and processes of a voltaic cell. 2.478 g of white phosphorus was used to make phosphine according to the equation: P 4 (s) +3OH − (aq)+3H 2 O (l) → PH 3 (g)+3H 2 PO 2− (aq) (i) Calculate the amount, in mol, of white phosphorus used. (ii) This phosphorus was reacted with 100.0 cm 3 of 5.00 mol dm −3 aqueous sodium hydroxide. A Voltaic Cell (also known as a Galvanic Cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. It consists of two separate half-cells . A half-cell is composed of an electrode (a strip of metal, M) within a solution containing M n+ ions in which M is any arbitrary metal. Label the diagram according to the components and processes of a voltaic cell. Consider the molecular view of an electrochemical cell involving the overall r. If a copper zinc voltaic cell utilizes znso4 and cuso4 solution you will use a saturated na2so4 solution in the salt bridge. Draw a diagram for this Galvanic cell, labeling the electron flow, the anode and cathode, and the positive and negative sides of the Galvanic cell. ... ("An")"ode"# of a cell, voltaic or electrochemical, attracts anions (ions with negative charges) and thus must carries some positive charges. So it is losing electrons and thus undergoing oxidation.
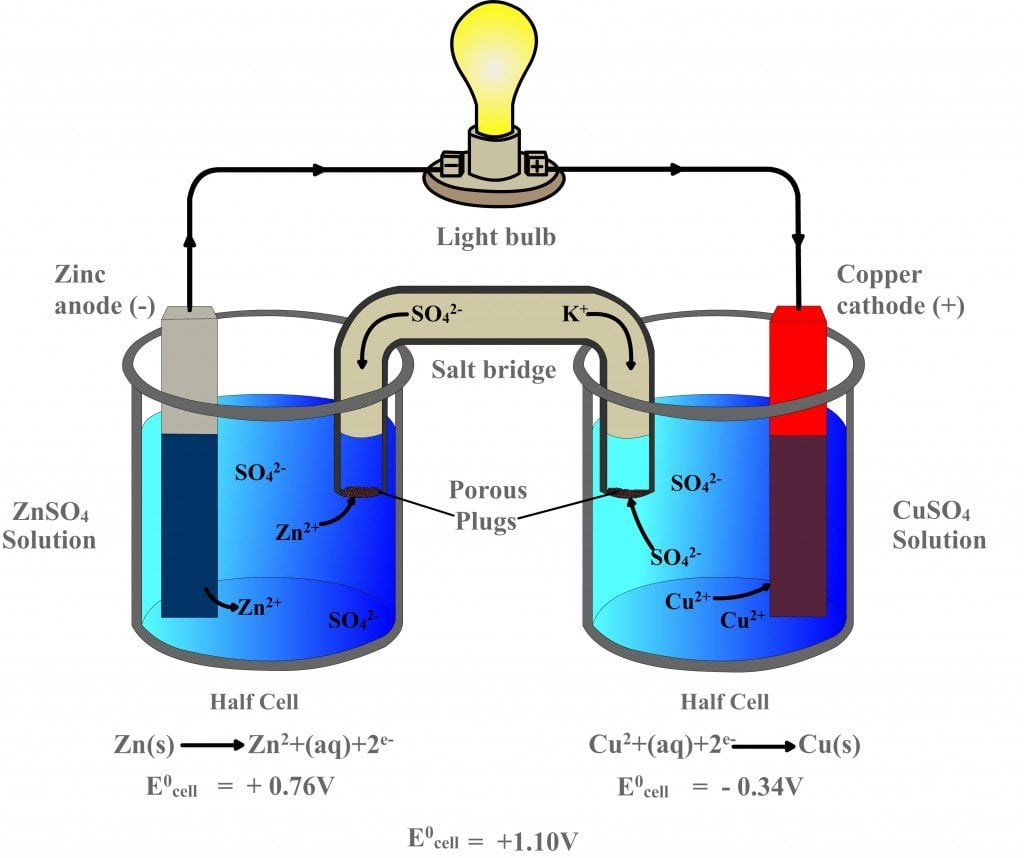
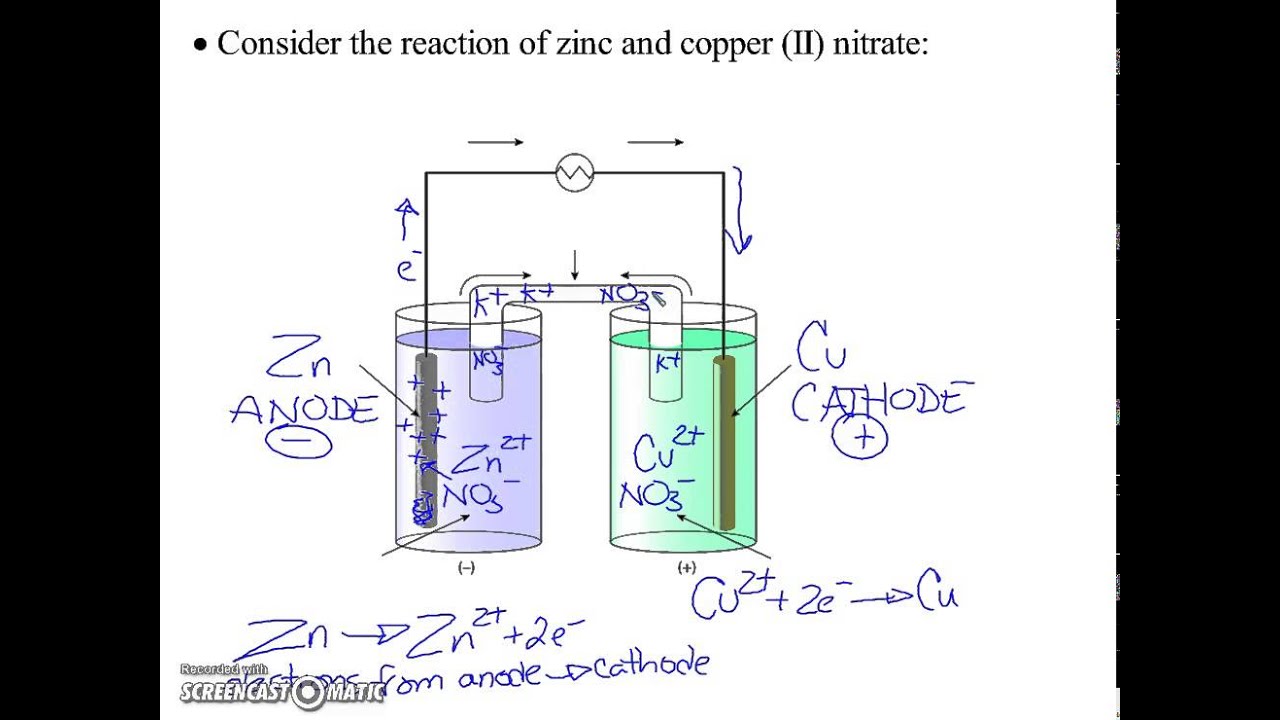
Label the diagram according to the components and processes of a voltaic cell. Drag the appropriate label to their respective targets. Asked over 1 year ago • Chemistry → Galvanic Cell Calculate the standard cell potential produced by a voltaic cell consisting of a sodium electrode in contact with a solution of Na+ ions and a copper electrode in contact with a solution of Cu2+ ions -2,713V MCA e-- +2e- go - + Ot34ôV A voltaic cell is constructed using electrodes based on the following half reactions: a. b. Pb (aq) + Pb(s) E Label the diagram according to the components and processes of a voltaic cell. Label the diagram according to the components and processes of a voltaic cell. Categories Uncategorized. Leave a Reply Cancel reply. Your email address will not be published. Required fields are marked * Sketch a cell diagram for the reaction. Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s) Solution: A. Draw one of the diagrams above (no labels). B. Identify what is oxidized and reduced. Zn is oxidized; Cu²⁺ is reduced. C. Put the oxidation materials in the left hand cell. Label the Zn as the negative anode. In the solution put Zn²⁺ and NO₃⁻.
Diagram and Working of an Electrolytic Cell Molten sodium chloride (NaCl) can be subjected to electrolysis with the help of an electrolytic cell, as illustrated below. Here, two inert electrodes are dipped into molten sodium chloride (which contains dissociated Na + cations and Cl - anions). A voltaic cell stable enough to be used as a battery is called a Daniell cell. For our purposes, we will work with the idealized Daniell cell in the figure below. We can use the known values of the standard-state reduction potentials for the Cu/Cu 2+ and Zn/Zn 2+ half-cells to predict the overall potential for the Daniell cell and to determine ... Wikipedia sayings about label the diagram according to the components and processes of a voltaic cell. drag the appropriate labels to their respective targets. 1.Glossary of engineering. inventor of the voltaic pile, the first electrical battery. In common usage, the word "battery" has come to include a single galvanic cell, but a battery Concept. Concept: Example: Problem: Label the diagram according to the components and processes of a voltaic cell. Drag the appropriate labels to their respective targets. FREE Expert Solution. 80% (385 ratings) play-rounded-fill. play-rounded-outline.
Solved Label the diagram according to the components and | Chegg.com. Science. Chemistry. Chemistry questions and answers. Label the diagram according to the components and processes of a voltaic cell. Drag the appropriate labels to their respective targets. Question: Label the diagram according to the components and processes of a voltaic cell.
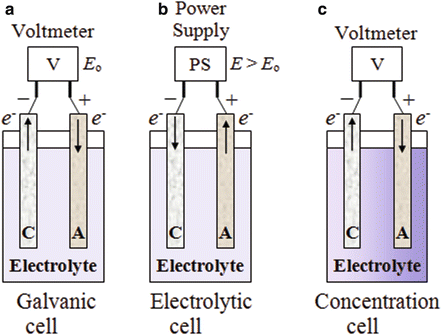
The voltaic cell and the electrolytic cell are both electrochemical cells which are at the heart of electrochemistry. In this ScienceStruck article, we learn about the individual workings of each of these cells, and then do a voltaic cell vs. electrolytic cell comparison by listing out their similarities and differences.
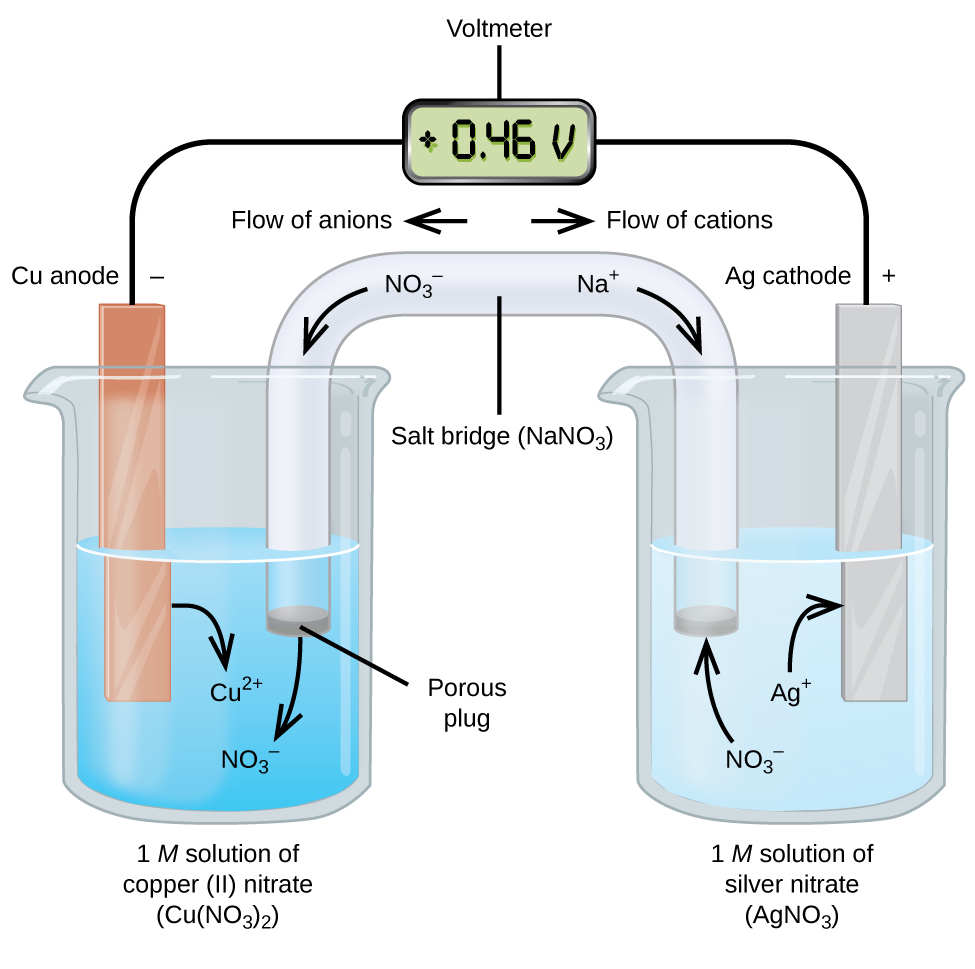
CH 142 Spring 2012 4 2. For the following voltaic cell Pb !Pb+2 (1 M) !! Cu+2 (1 M) !Cu for which the following data were obtained, determine ΔG at each temperature, then graph ΔG versus absolute T. Find the ΔH value for this battery and the ΔS value using the graph created. Note that while there can be several values for ΔG there is just one value for ΔH and
Basic voltaic cell diagram In order to draw any voltaic cell, we need each of these elements in the basic diagram. We will need to know what goes into the oxidizing side and on the reducing side of...
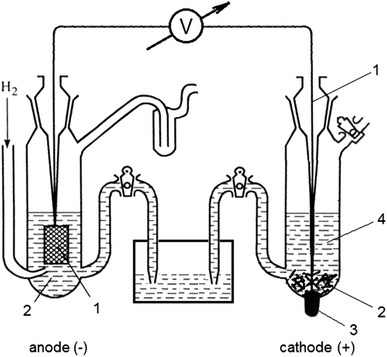
Label the diagram according to the components and processes of a voltaic cell. The half-cell is a chamber in the voltaic cell where one half-cell is the site of the oxidation reaction and the other half-cell is the site of the reduction reaction. Type the half-cell reaction that takes place at the anode for the cobalt-silver voltaic cell.
Label the diagram according to the components and processes of a voltaic cell. This creates a current of electrons moving across a wire that can be used for energy. Electrolytes containing the ions involved in the redox reaction. Type the half cell reaction that takes place at the anode for the cobalt silver voltaic cell.
Muscle cell s form tissue that creates . The orbital diagram for a groundstate oxygen a to m is A A B B C C D D E E Which element has A galvanic voltaic cell consists of an electrode Crossing the bridge between the rmodynamics and. Untitled. Hybrid silicon nanowire devices and the ir functional. 34 label the diagram according to the...
Label each half cell in this diagram of a voltaic cell to identify which electrode it contains and the process… Get the answers you need, now! DominiqueT00 DominiqueT00 04/21/2020 Chemistry Middle School answered Label each half cell in this diagram of a voltaic cell to identify which electrode it contains and the process occurring at the ...
Principle of Galvanic (Voltaic) Cell. Electric work done by a galvanic cell is mainly due to the Gibbs energy of spontaneous redox reaction in the voltaic cell. It generally consists of two half cells and a salt bridge. Each half cell further consists of a metallic electrode dipped into an electrolyte. These two half-cells are connected to a ...
A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction () to generate electricity. This type of electrochemical cell is often called a voltaic cell after its inventor, the Italian physicist Alessandro Volta (1745-1827). In contrast, an electrolytic cell consumes electrical energy from an external source, using ...
Chapter 17: Electrochemistry. Label the diagram according to the components and processes of a voltaic cell. The half-cell is a chamber in the voltaic cell where one half-cell is the site of the oxidation reaction and the other half-cell is the site of the reduction reaction. Type the half-cell reaction that takes place at the anode for the ...
Electrolytic Cells. Voltaic cells use a spontaneous chemical reaction to drive an electric current through an external circuit. These cells are important because they are the basis for the batteries that fuel modern society. But they aren't the only kind of electrochemical cell. It is also possible to construct a cell that does work on a ...
Answer to label the diagram according to the components and processes of a voltaic cell. A voltaic cell has several components o o o o o o anode. Both types of cells use two electrodes that provide an electrical connection between systems that are separated in space. Block Diagram Of The Proposed Smart Rfid Label Download
A voltaic cell is made from magnesium and iron half-cells. Magnesium is a more reactive metal than iron. Which statement is correct when the cell produces electricity? A. Electrons are lost from Magnesium atoms. B. The concentration of Fe 2+ ions increases. C. Electrons flow from the iron half-cell to the Magnesium half-cell.


















0 Response to "40 label the diagram according to the components and processes of a voltaic cell"
Post a Comment