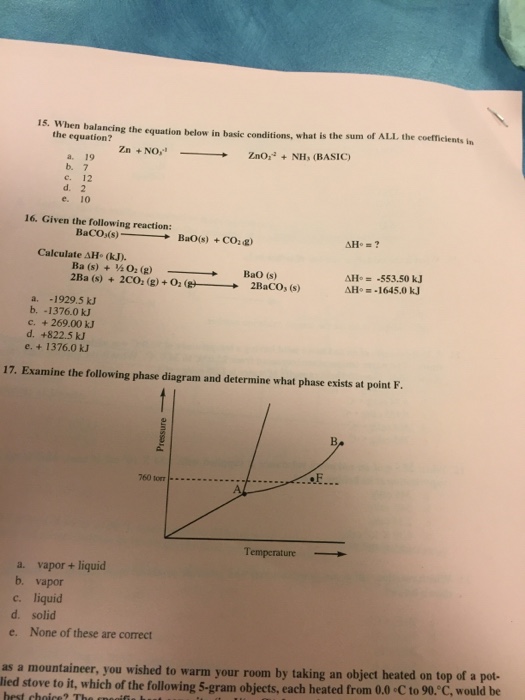
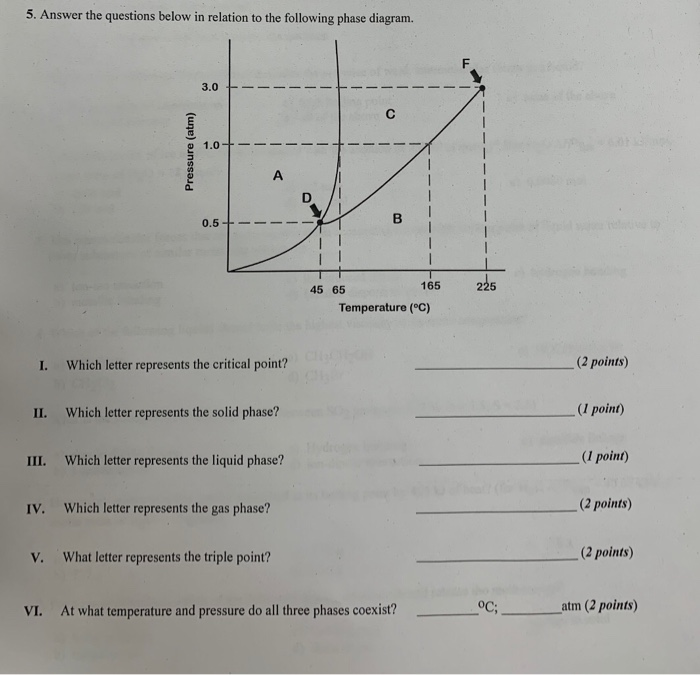
39 examine the following phase diagram and determine what phase exists at point c.
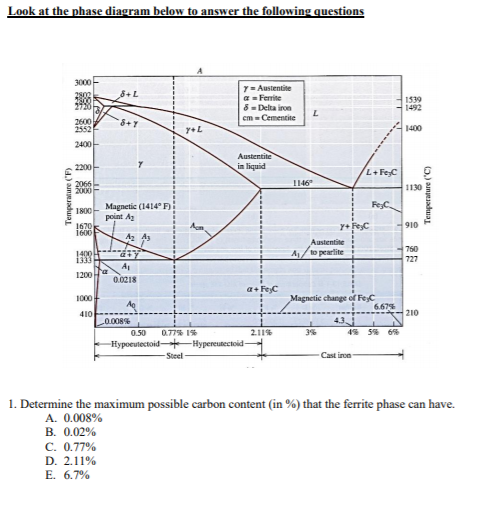
• Phase Diagrams Phase diagrams are graphs that give information on the equilibrium temperature and pressure for a particular compound. The equilibria occur for the solid- liquid plateau, liquid-vapor plateau and solid-vapor plateau. In this experiment, the phase diagram is shown for the solid-liquid equilibrium point, and varies from 100% ... 3C) phase diagram Ferrite-α-BCC, low C solubility(0.022%wt), magnetic ... below the eutectoid, determine the following a) composition of Fe 3C and ferrite (α) b) the amount of carbide (cementite) in grams that forms per 100 g of steel ... 3C phases 120 μm γ γ ...
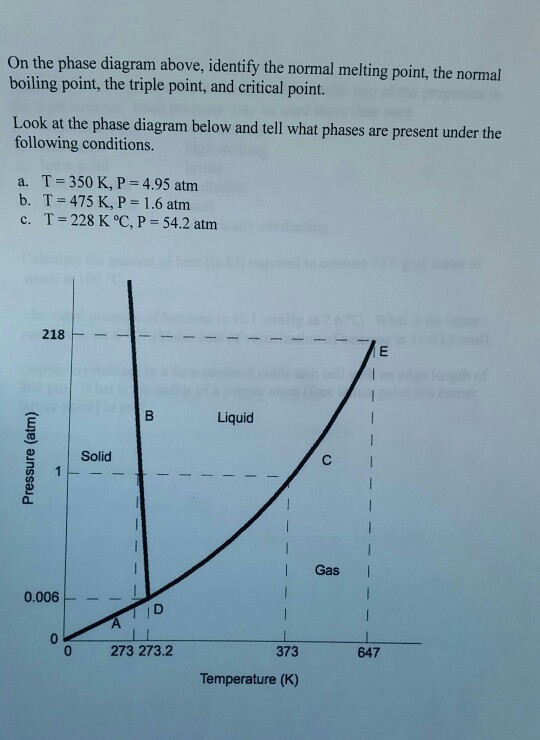
• A phase diagram of a pure compound has a triple point at 13 °C and 205 mmHg, a normal melting point at 17 °C, and a normal boiling point at 87 °C. Draw a phase diagram for this compound. Label all the different regions of the phase diagram. Marks 7 Indicate whether each of the following statements regarding this compound is true or false.
Examine the following phase diagram and determine what phase exists at point c.
60 seconds. Q. In an experiment, a scientist burned 10.0 g of methane, and the temperature of the 500.0 mL of water in the calorimeter changed from 21.0°C to 26.6°C. The specific heat of water is 4.18 J/g·°C. How much energy was released in joules? answer choices. 5.00 x 10^3 J. In Figure \(\PageIndex{2b}\) point A is located at P = 1 atm and T = −1.0°C, within the solid (ice) region of the phase diagram. As the pressure increases to 150 atm while the temperature remains the same, the line from point A crosses the ice/water boundary to point B, which lies in the liquid water region. 3-Dimensional Depiction of Temperature-Composition Phase Diagram of Bismuth, Tin, and Lead at 1atm. The diagram has been simplified by omission of the regions of solid solubility. Each face of the triangular a eutectic. There is also a peritectic point in the Bi-Pb phase diagram. Figure by MIT OCW.
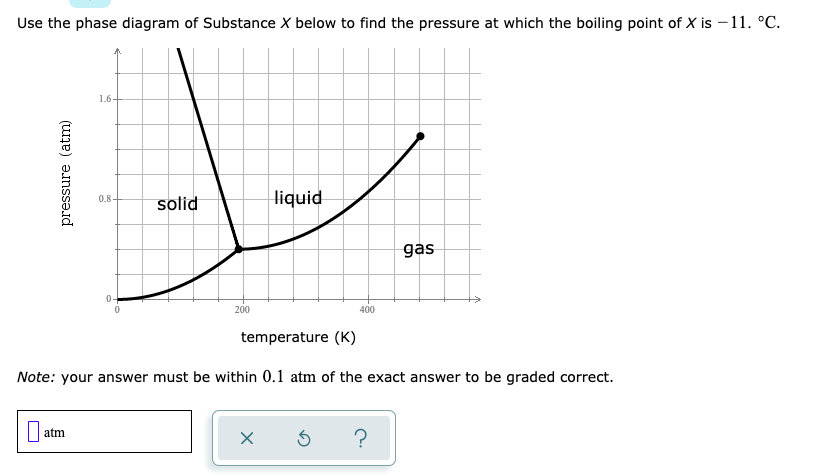
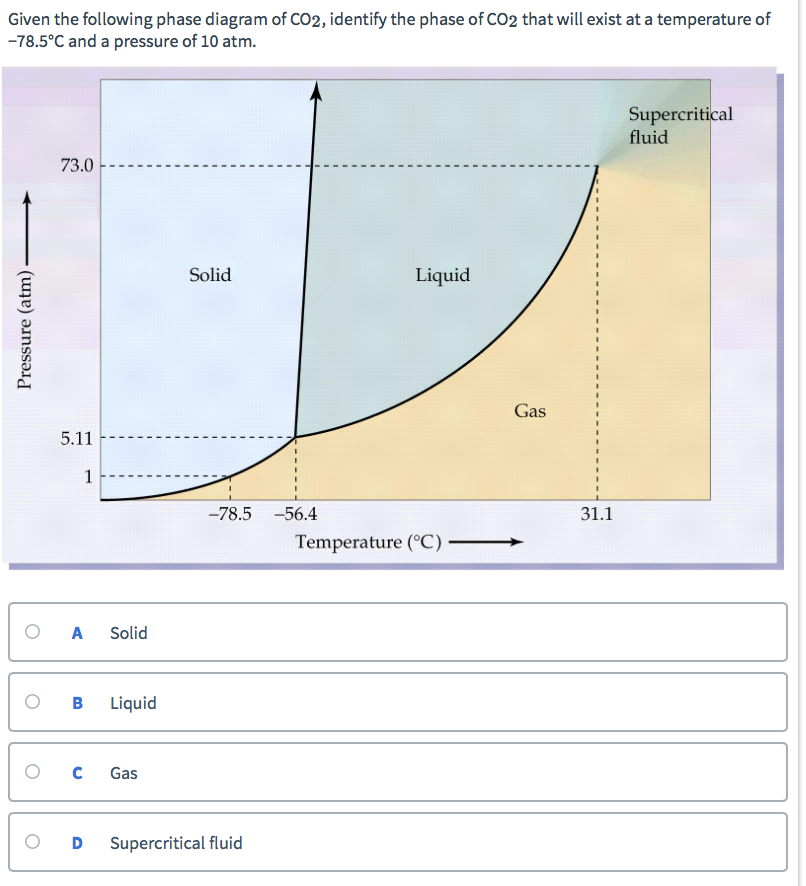
Examine the following phase diagram and determine what phase exists at point c.. Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash Determining the State of Carbon Dioxide Using the phase diagram for carbon dioxide shown in (Figure), determine the state of CO 2 at the following temperatures and pressures: (a) −30 °C and 2000 kPa. (b) −60 °C and 1000 kPa. (c) −60 °C and 100 kPa. (d) −40 °C and 1500 kPa. (e) 0 °C and 100 kPa. (f) 20 °C and 100 kPa. mass fraction of each phase is 0.5, estimate: (a) The temperature of the alloy (b) The compositions of the two phases Solution (a) We are given that the mass fractions of α and liquid phases are both 0.5 for a 30 wt% Sn-70 wt% Pb alloy and asked to estimate the temperature of the alloy. Using the appropriate phase diagram, Figure 9.8, by trial Imagine a substance with the following points on the phase diagram: a triple point at .5 atm and -5°C; a normal melting point at 20°C; a normal boiling point at 150°C; and a critical point at 5 atm and 1000°C. The solid liquid line is "normal" (meaning positive sloping). For this, complete the following: 1.
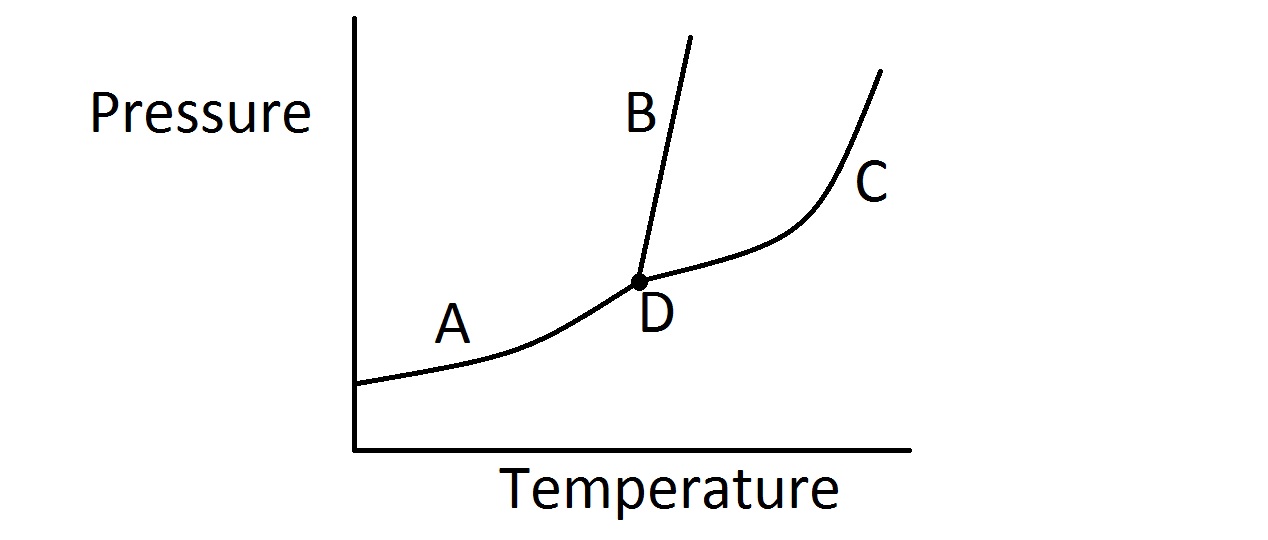
Phase Diagrams. The figure below shows an example of a phase diagram, which summarizes the effect of temperature and pressure on a substance in a closed container. Every point in this diagram represents a possible combination of temperature and pressure for the system. The diagram is divided into three areas, which represent the solid, liquid ... A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures ... substance. At point B, the temperature of the substance is 70°C. The solid begins to melt. At point C, the substance is completely melted or in a liquid state. Material in this phase has indefinite shape and definite volume. The energy put to the substance between minutes 5 and 9 was used to convert the substance from a solid to a liquid. Examine the following phase diagram and identify the feature represented by point A. A. melting point B. critical point C. triple point D. sublimation point E. boiling point 2. Examine the following phase diagram and determine what phase exists at point F. A. vapor + liquid B. vapor C. liquid D. solid E. supercritical fluid
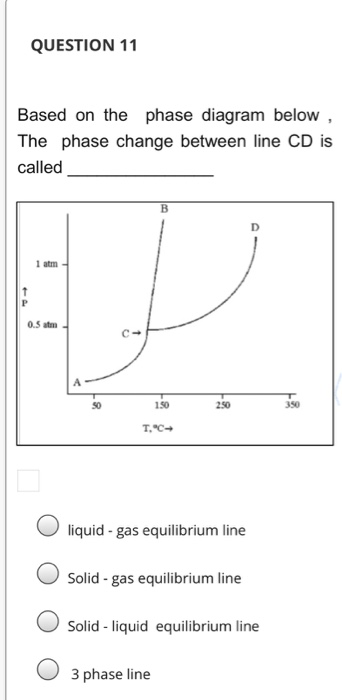
Peritectic point - The point on a phase diagram where a reaction takes place between a previously precipitated phase and the liquid to produce a new solid phase. When this point is reached, the temperature must remain constant until the reaction has run to completion. A peritectic is also an invariant point. Intermediate compound - A phase that ... Point B is in the liquid phase and Point C is in the gas phase. The lines on a phase diagram correspond to the dividing lines between two phases. These lines are known as phase boundaries. At a point on a phase boundary, the substance can be in either one or the other phases that appear at either side of the boundary. Question: Examine the following phase diagram and determine what phase exists at point 760 som Temperature A) supercritical fluid B) liquid C) vapor+liquid D) vapor E) solid The phase diagram of a substance i s given below. This substance is a at 25°C and 1.0 atm. 1.5 P (atm) 1.0 0.5T -10 0 10 20 30 40 50 60 70 T ('C) A) gas B) crystal C ... The Cu-Ni phase diagram (Figure 9.3a) is shown below; the point labeled "G" represents the 63.8 wt% Ni-36.2 wt% Cu composition at 1250°C. As may be noted, point G lies within the α phase field. Therefore, only the α phase is present; its composition is 63.8 wt% Ni-36.2 wt% Cu.
Question. : 12) Examine the following phase diagram and identify the feature represented by point A. 760 torr Temperature A) melting point B) critical point C) triple point D) sublimation point E) boiling point 13) In hydrogen iodide are the most important intermolecular forces A) B) C) dipole-dipole forces London dispersion forces hydrogen ...
Chapter 12-Consider the following phase diagram and identify the process occurring as one goes from point C to point D. Increasing temperature with a phase change from solid to vapor -Examine the following phase diagram and determine what phase exists at point F. Vapor
(a) 0 (b) 1 (c) 2 (d) 3 4. Above the following line, liquid phase exist for all compositions in a phase diagram. (a) Tie-line (b) Solvus (c) Solidus (d) Liquidus 5. Following is wrong about a phase diagram. (a) It gives information on transformation rates. (b) Relative amount of different phases can be found under given equilibrium conditions.
Example: Phase Equilibria For a 99.6 wt% Fe-0.40 wt% C at a temperature just below the eutectoid, determine the following a) composition of Fe 3 C and ferrite (a) b) the amount of carbide (cementite) in grams that forms per 100 g of steel c) the amount of pearlite and proeutectoid ferrite (a) 32
We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. For example, a pressure of 50 kPa and a temperature of −10 °C correspond to the region of the diagram labeled “ice.”. Under these conditions, water exists only as a solid (ice).
Examine the following phase diagram and determine what phase exists at point F Vapor When the electron cloud of a molecule is easily distorted, the molecule has a high _____________.
Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ...
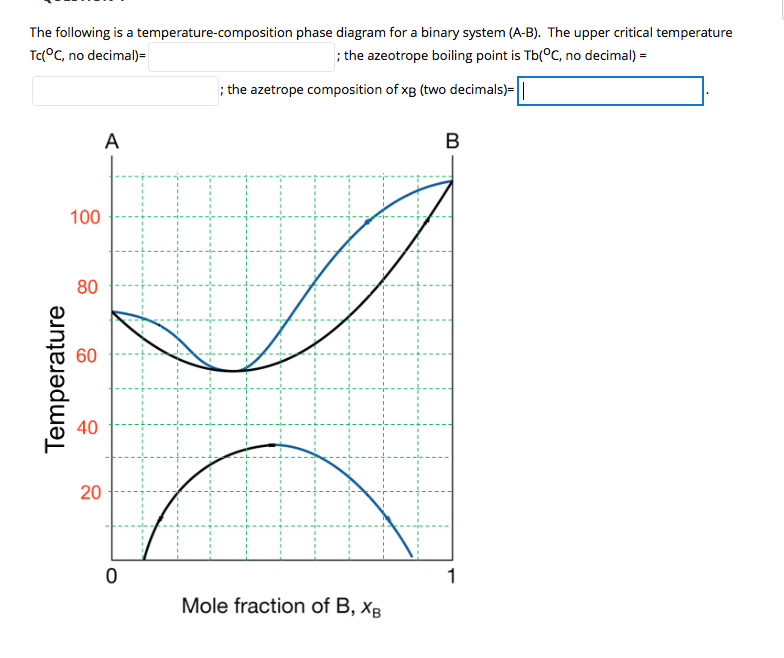
as axes are called phase diagrams. DEF. A phase diagram (or equilibrium diagram) is a diagram with T and composition as axes, showing the equilibrium constitution. The phase diagram of an alloy made of components A and B, for all combinations of T and X B, defines the A-B system. Binary systems have two components, ternary systems three, and so on.
1). Phase diagrams are useful tools to determine:-the number and types of phases, the wt% of each phase and the composition of each phase for a given T and composition of the system. 2). Binary eutectics and binary eutectoids allow for a range of microstructures with different properties
Examine the following phase diagram and identify the feature represented by point A. ... Consider the following phase diagram and identify the process occurring as one goes from point C to point D. ... Examine the following phase diagram and determine what phase exists at point F. SEE QUESTION 14 Image A) vapor + liquid B) vapor
(A) 10.1 kJ (B) 13.1 kJ (C) 16.1 kJ (D) 48.6 kJ 38. Examine the following phase diagram and identify the feature represented by point A. A. melting point B. critical point C. triple point D. sublimation point 39. What are the changes in phase going from points A to B to C to D A B D C T P
3-Dimensional Depiction of Temperature-Composition Phase Diagram of Bismuth, Tin, and Lead at 1atm. The diagram has been simplified by omission of the regions of solid solubility. Each face of the triangular a eutectic. There is also a peritectic point in the Bi-Pb phase diagram. Figure by MIT OCW.
In Figure \(\PageIndex{2b}\) point A is located at P = 1 atm and T = −1.0°C, within the solid (ice) region of the phase diagram. As the pressure increases to 150 atm while the temperature remains the same, the line from point A crosses the ice/water boundary to point B, which lies in the liquid water region.
60 seconds. Q. In an experiment, a scientist burned 10.0 g of methane, and the temperature of the 500.0 mL of water in the calorimeter changed from 21.0°C to 26.6°C. The specific heat of water is 4.18 J/g·°C. How much energy was released in joules? answer choices. 5.00 x 10^3 J.



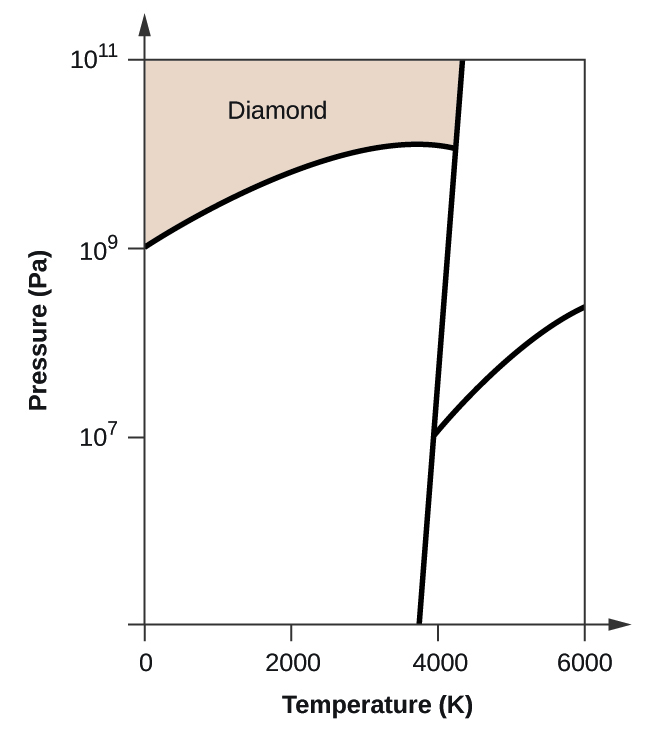
![[Solved] Use the accompanying phase diagram for carbon to ...](https://s3.amazonaws.com/si.question.images/image/images7/442-C-P-c-L-A-S(86).png)
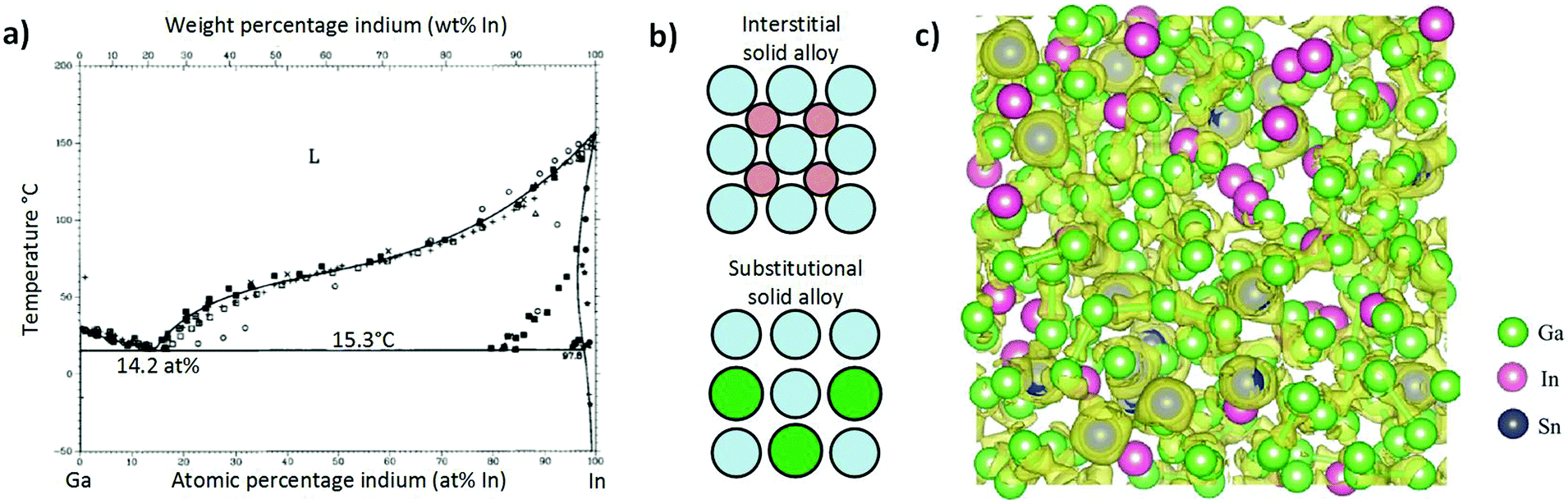













0 Response to "39 examine the following phase diagram and determine what phase exists at point c."
Post a Comment