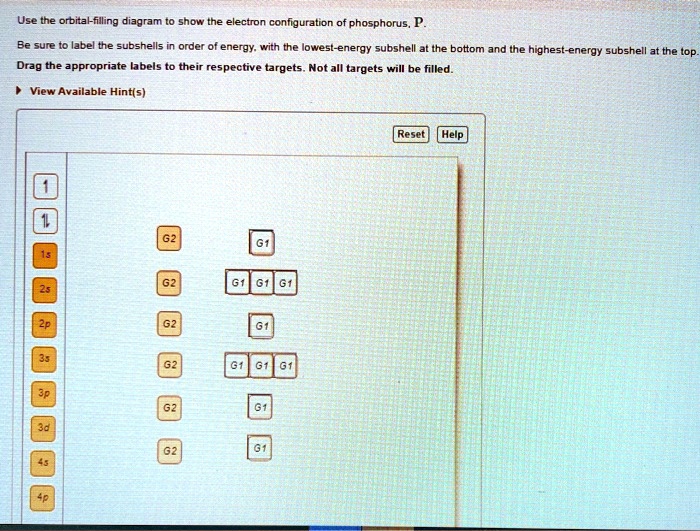
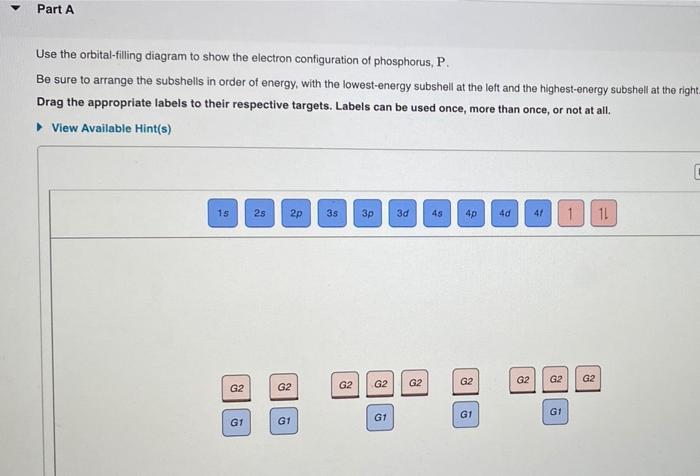
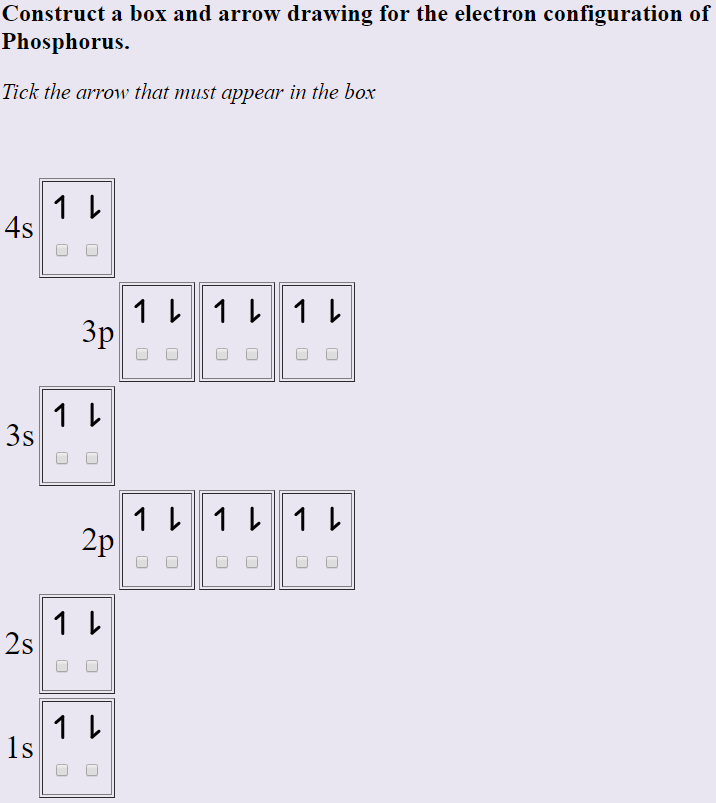
38 use the orbital-filling diagram to show the electron configuration of phosphorus, p.
Which element is represented by the ground state electron configuration 1s22s22p4 The electron configuration for Si is 1s22s22p63s23p2. From this method of writing the electron configuration, you cannot predict the number of unpaired electrons. You can write the electron configuration as an orbital diagram as (see picture)...
Use the orbital-filling diagram to show the electron configuration of phosphorus P Orbital Diagrams: Electronic configuration is the distribution of electrons in the atomic orbitals like s,p,d,f,g.

Use the orbital-filling diagram to show the electron configuration of phosphorus, p.
For the CH3CH2NH2 structure use the periodic table to find the total May 30, 2021 · A lewis dot structure is also called a lewis structure, a lewis dot diagram, an electron dot structure note the lone pair (dots without bonds) on top of p, just like for n in the previous example for nh3. Lewis structure of carbonate ion is drawn in this tutorial step by step. 7 Lewis Diagrams, Formal Charge ... Box spin diagram of outer electron orbitals for the electron configuration of the 15 Phosphorus P 1s22s22p63s23p3 Ne3s 3p P pblock Gp5 Build the orbital diagram for the ion most likely formed by phosphorus. The rules for orbital filling diagrams. Energy 0 1 1 x 5. Now this is only one way we can draw the electron dot diagram for Oxygen. Draw ... Orbital filling elected ements Electron 3s configuration Isl C] element (answer) en on Element O Ne 2Px 2py 2pz 2. Which element has the following orbital diagram? 3. Using arrows, show how the following orbitals will fill with electrons. E?ecrron Configurayion Is 2s Si s--s--p-šs- Is: 2s22pt3s23p- I 4.
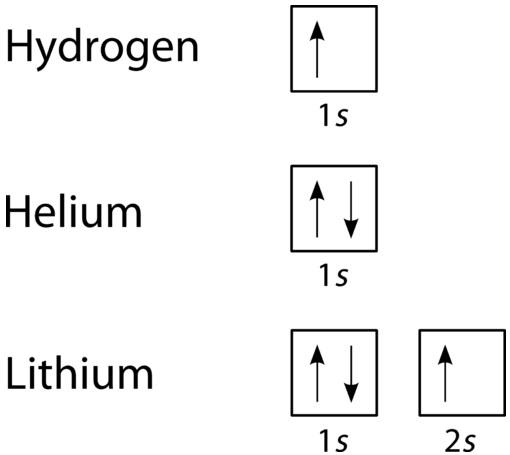
Use the orbital-filling diagram to show the electron configuration of phosphorus, p.. 30 May 2020 — The electron configuration of an atom indicates the number of valence ... We can clearly see that p orbitals are half-filled as there are ... The electron configuration for Si is 1s22s22p63s23p2. From this method of writing the electron configuration, you cannot predict the number of unpaired electrons. You can write the electron configuration as an orbital diagram as (see picture)... Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2 s orbital (Figure 8.3. 3 or 8.3. 4 ). Thus, the electron configuration and orbital diagram of lithium are:
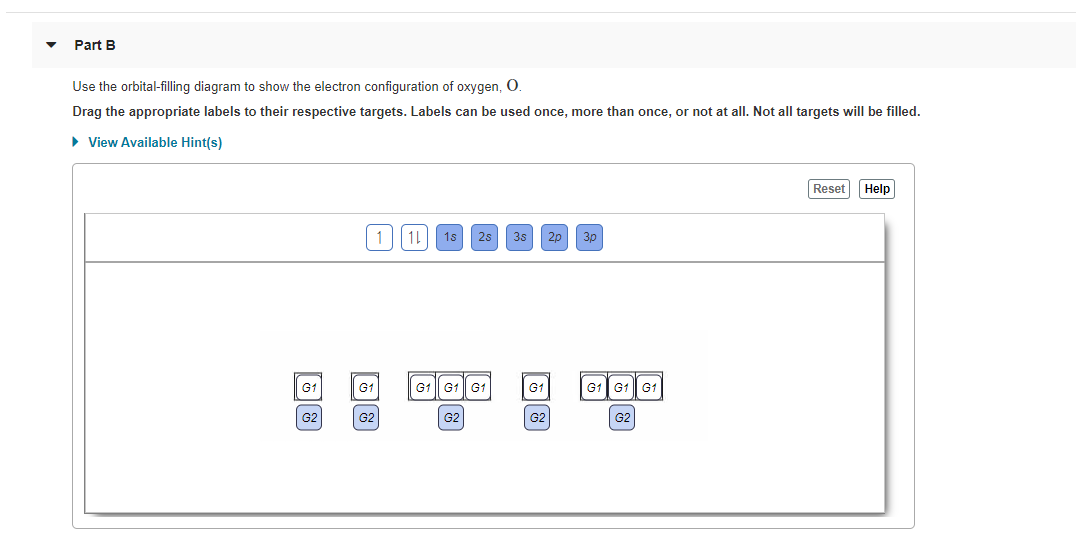
The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of: 21 Jan 2021 — Phosphorous is a chemical element that has an atomic number of 15. Its electron configuration with regards to electrons present in each shell is ... Transcribed image text: Use the orbital filling diagram to show the electron configuration of phosphorus, P. Be sure to label the subshells in order of ... The electron configuration for oxygen is: 1s^2 2s^2 2p^4 This video will walk you through the step of writing orbital diagram. The video uses Kr as an example, but the process is exactly as the same as what you need to do for oxygen. Hope this helps!
You are watching: Use the orbital-filling diagram to show the electron configuration of phosphorus, p. Electron Configuration. Electron configurations are the summary of where the electrons are around a nucleus. As we learned earlier, each neutral atom has a number of electrons equal to its number of protons. 30.11.2021 · Draw a box diagram to show the electron configuration of oxygen. Therefore, NF orbital will contain 1 electron before a single orbital will contain 2 electrons –The three p orbitals fill in the order shown: •The number of arrows must match the number of electrons contained in the atom Orbital Filling Diagrams 5. Build an atom out of protons, neutrons, and electrons, and see how the element ... The order of filling of the energy levels is 1 s, 2 s, 2 p, 3 s, 3 p, 4 s, . . . The 15 electrons of the phosphorus atom will fill up to the 3 p orbital, which will contain three electrons: The last electron added is a 3 p electron. Therefore, n = 3 and, for a p -type orbital, l = 1. The ml value could be -1, 0, or +1. 23.11.2021 · Which of the following is the correct orbital diagram for a nitrogen (n) atom_
The key difference between orbital diagram and electron configuration is that the orbital diagram shows the electrons in arrows, indicating the spin of electrons. But, the electron configuration does not show details on the spin of electrons. The orbital diagram shows the arrangement of the electrons given by the electron configuration.
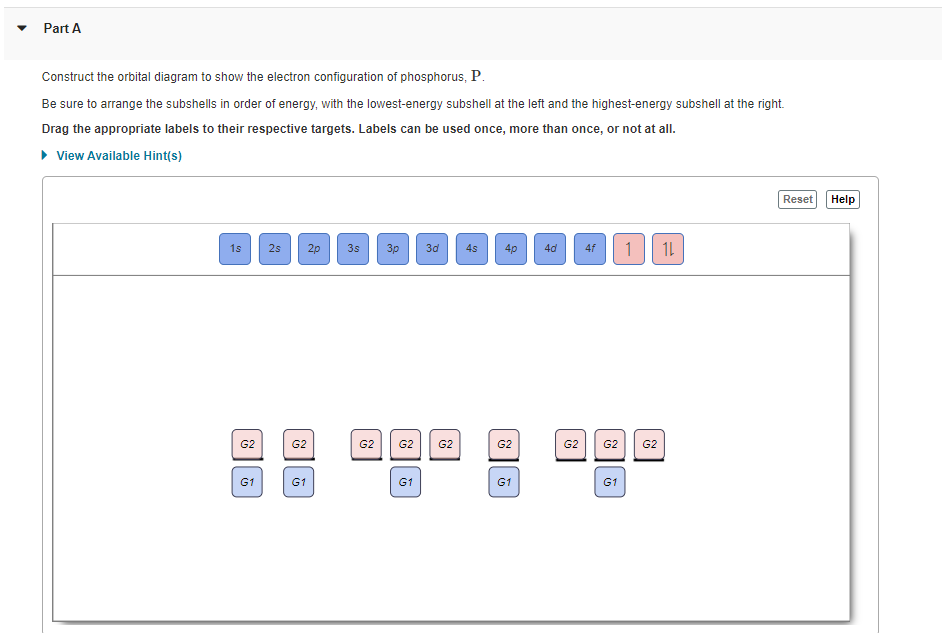
Use the orbital-filling diagram to show the electron configuration of helium, He. ... Enter an abbreviated electron configuration for phosphorus: Express your answer in complete form, in order of increasing energy. [Ne] 3s2 3p3. Enter an abbreviated electron configuration for arsenic:
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
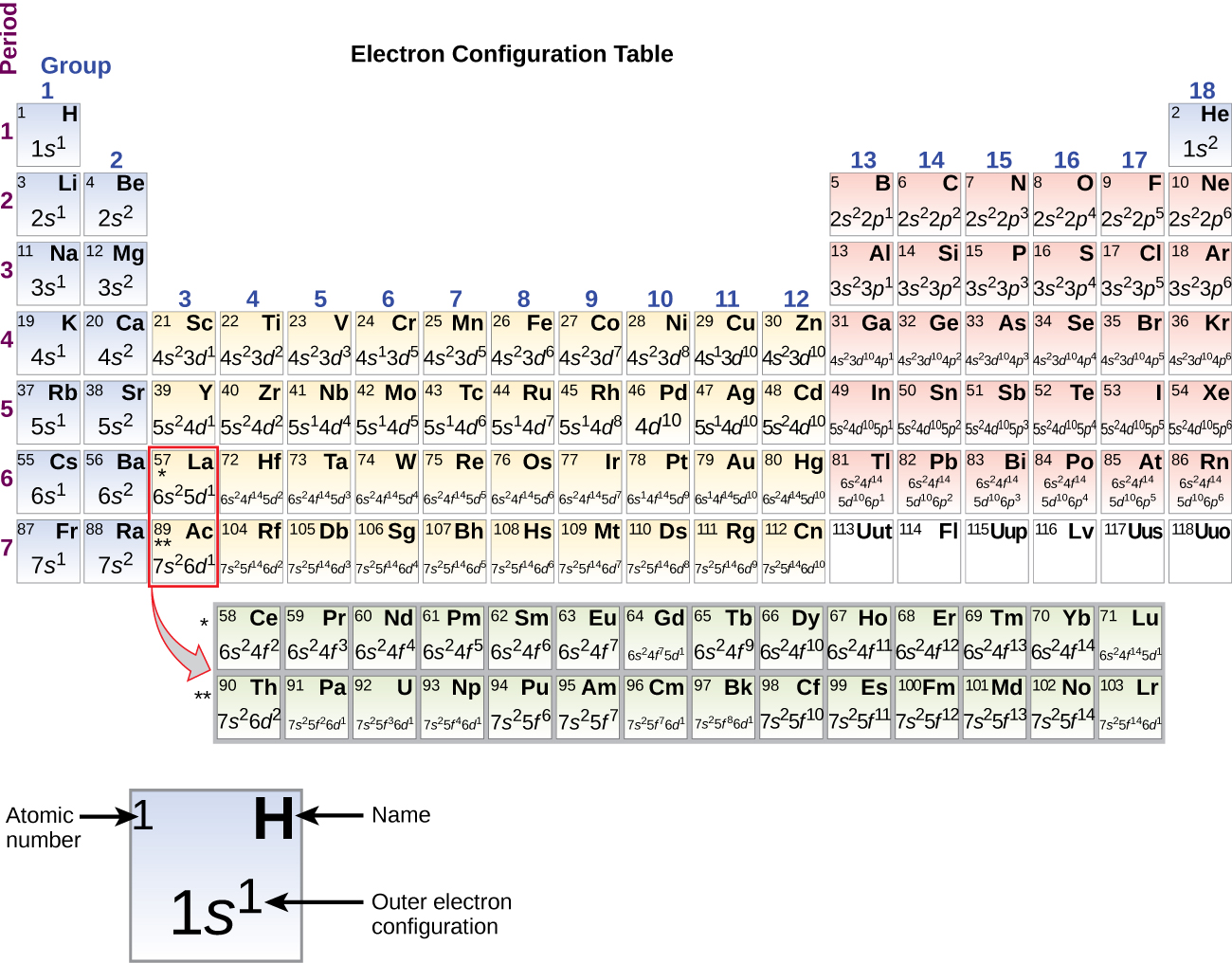
Physicists and chemists use a standard notation to indicate the electron configurations of atoms and molecules. For atoms, the notation consists of a sequence of atomic subshell labels (e.g. for phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. For example, hydrogen has one electron in the s-orbital of the first shell ...
Start studying Bohr Model, Electron Config, and Orbital Diagrams. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
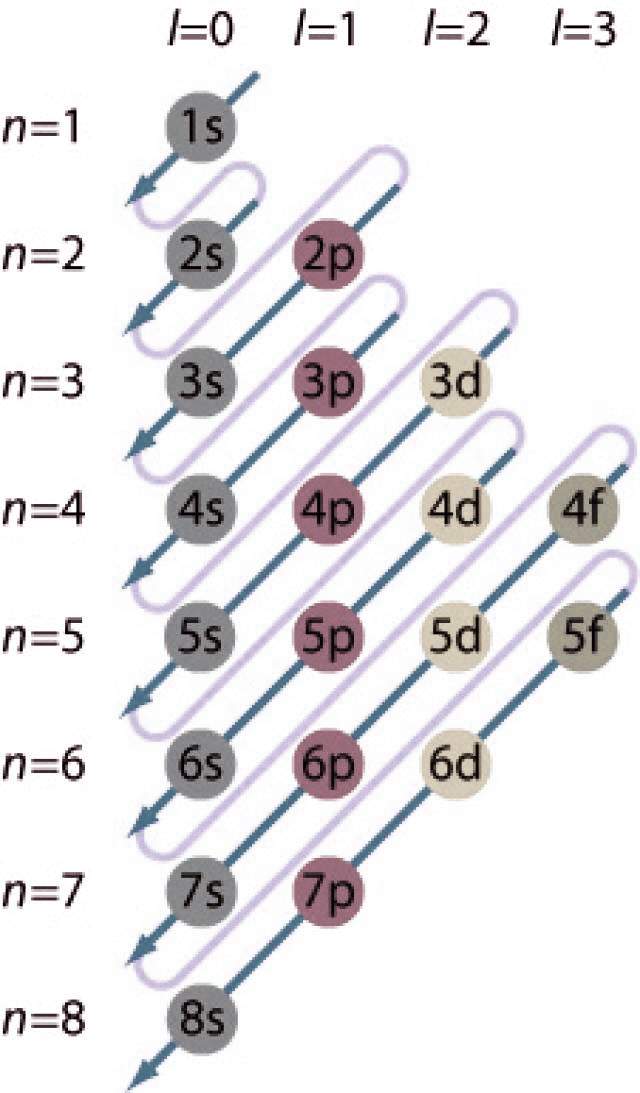
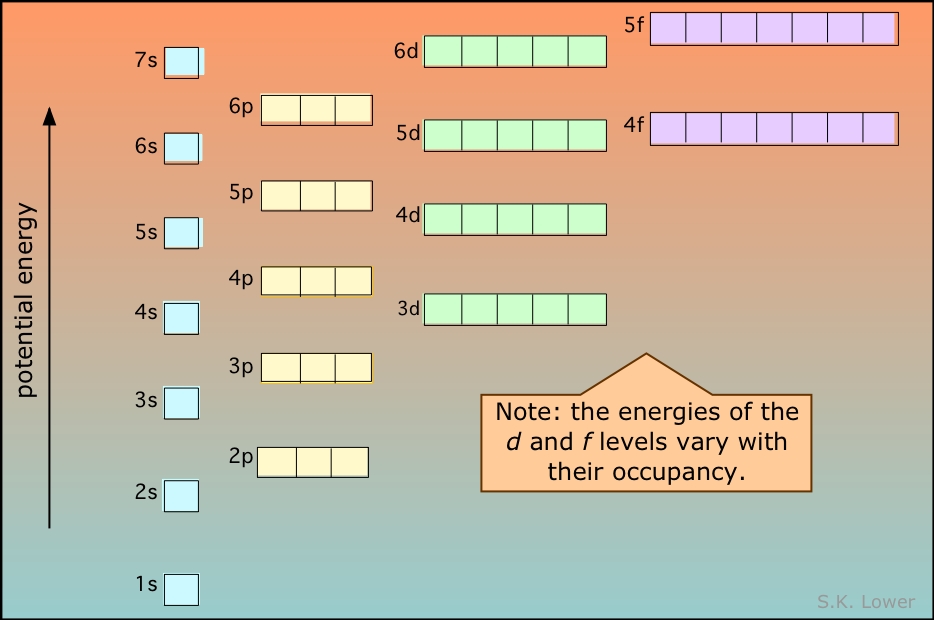
For example, after filling the 3p block up to Ar, we see the orbital will be 4s (K, Ca), followed by the 3d orbitals. This figure includes a chart used to order ...
Orbital Diagram, electron configuration, and the noble gas notation for a zinc (Zn) atom.
Draw the orbital filling diagram for carbon and write its electron configuration. Solution. Step 1: List the known quantities and plan the problem. Known. Atomic number of carbon, \(Z=6\) Use the order of fill diagram to draw an orbital filling diagram with a total of six electrons. Follow Hund's rule. Write the electron configuration.
The electron configuration for Gallium, Ga is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^1 Gallium, Ga has 31 protons and 31 electrons. The superscripts represent the electrons present in each region of the periodic table. The sum of these superscripts should equal the atomic number for a neutral atom. The last electron is in the 4th period, in the p region and the first electron in that region.
Nitrogen atom model
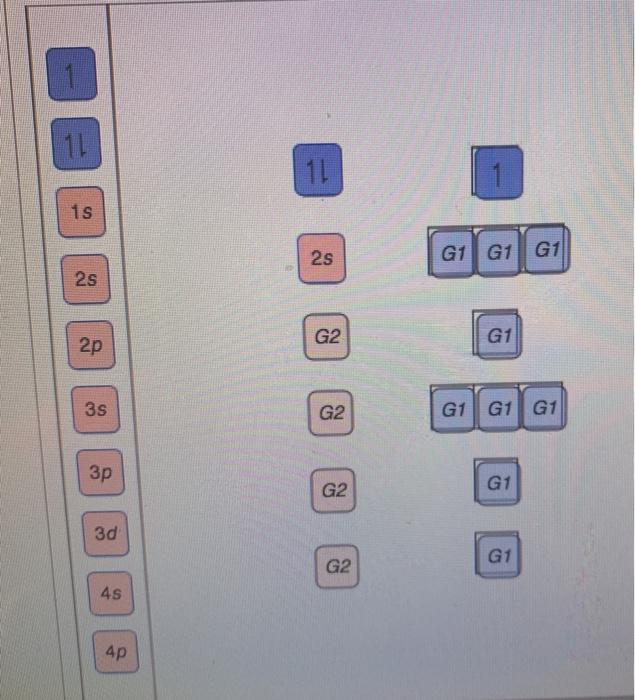
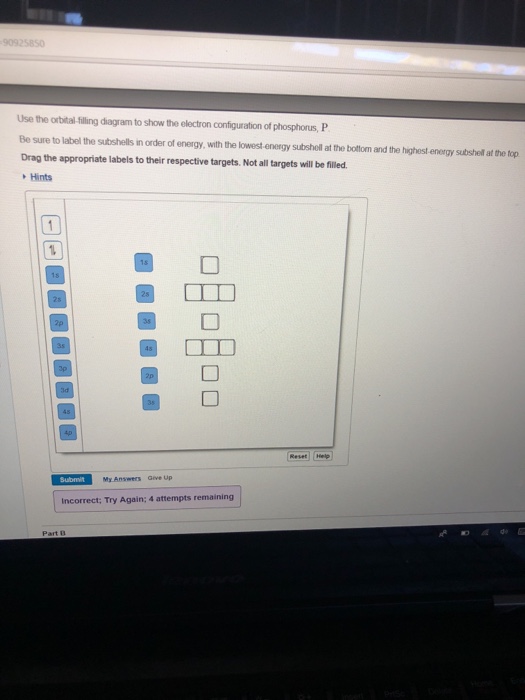
Use the orbital-filling diagram to show the electron configuration of phosphorus, P. Be sure to label the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the Drag the appropriate labels to their respective targets. Not all targets will be filled.
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ).
Part A Use the orbital-filling diagram to show the electron configuration of phosphorus, P. Be sure to arrange the subshells in order of energy, with the lowest-energy subshell at the left and the highest-energy subshell at the right Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. ...
An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to represent the electrons in ...
Orbital Filling … Orbital diagram Electron configuration E. Worksheet 4 - Electron Configurations. 2019 Element Builder Answer Key Vocabulary. Symbol # e-Orbital Notation your answer for each. The valence orbital is the highest, outermost orbital of the atom and is the only one involved in the bonding of the atom. It is very useful in determining electron pairing and thus predicting ...
In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
Write out the complete electron configuration for a phosphorus atom. View Answer . How many p-orbitals are occupied in an Ne atom? View Answer. Match each element (Sr, Se, Cl, Kr, Rb, Si) with its ...
The electron configurations and orbital diagrams of these four elements are: The alkali metal sodium (atomic number 11) has one more electron than the neon atom. This electron must go into the lowest-energy subshell available, the 3 s orbital, giving a 1 s 2 2 s 2 2 p 6 3 s 1 configuration.
Determine the number of electrons in a neutral phosphorus atom Reset Help Chemistry: M. X ViewPassignment Problemid=127565898 ③ 10126 Constants Periodic Table Drag and drop orbitals and electron counts to complete the electron configuration of iron, Fe.
Orbital Filling Diagram for Nitrogen. Each bromine would donate one 4pz electron to form a σ -bonding orbital. magnesium D. Nov 05, 2019 · For that, we have electron shell diagrams. Beryllium 8. A more accurate Bohr model of a nitrogen atom could look like this: Example: Orbital diagram for Li 1s2 filled 2s1 half-filled 2p empty Order of Filling Electrons in an atom fill the lowest energy ...
1 answerThe atomic number of phosphorus is 15. It has 5 valence electrons in its valence shell. The orbitals involved in phosphorus atoms are 1s, 2s, 2p, 3s and 3p.
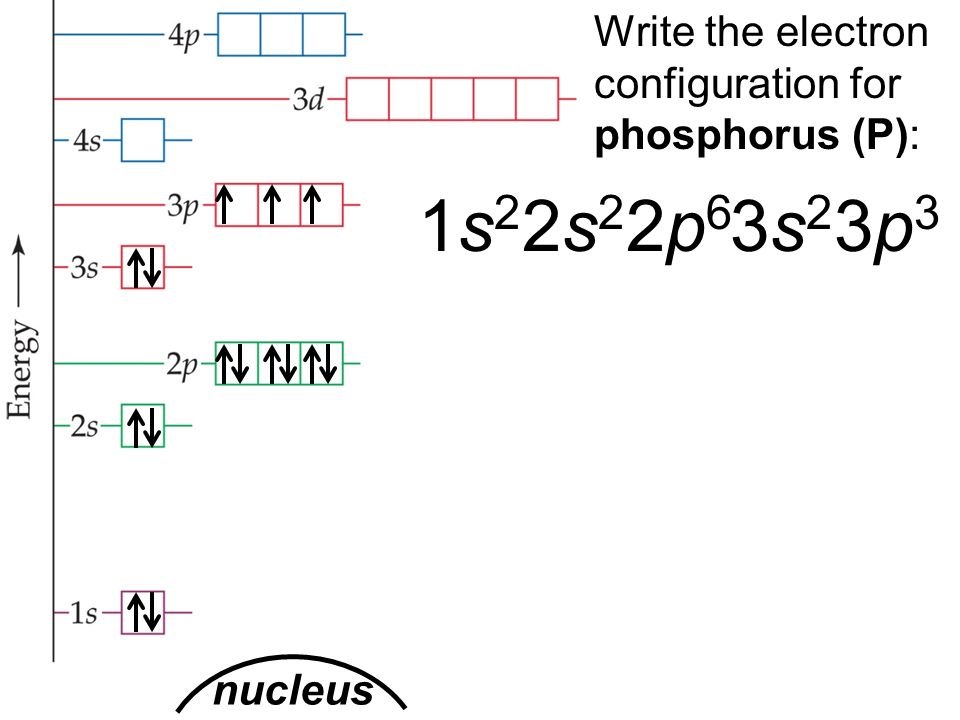
4 Jul 2020 — The electron configuration chart for phosphorus, P, is given as follows;. 1s²2s²2p⁶3s²3p. ... Phosphorus, with the 3 partially filled p orbitals ...1 answer · 10 votes: Answer:1) The full electron configuration for phosphorus is 1s²2s²2p⁶3s²3p2) Phosphorus is paramagneticExplanation:1) To write the electron configuration, ...
Electron Configurations. The content that follows is the substance of General Chemistry Lecture 26. In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the relationship of electron configuration to the periodic properties of the elements.
use the orbital-filling diagram to show the electron configuration of phosphorus, p. Answer + 20. Watch.
The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining electron. Therefore the Aluminium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 1.
Orbital filling elected ements Electron 3s configuration Isl C] element (answer) en on Element O Ne 2Px 2py 2pz 2. Which element has the following orbital diagram? 3. Using arrows, show how the following orbitals will fill with electrons. E?ecrron Configurayion Is 2s Si s--s--p-šs- Is: 2s22pt3s23p- I 4.
Box spin diagram of outer electron orbitals for the electron configuration of the 15 Phosphorus P 1s22s22p63s23p3 Ne3s 3p P pblock Gp5 Build the orbital diagram for the ion most likely formed by phosphorus. The rules for orbital filling diagrams. Energy 0 1 1 x 5. Now this is only one way we can draw the electron dot diagram for Oxygen. Draw ...
For the CH3CH2NH2 structure use the periodic table to find the total May 30, 2021 · A lewis dot structure is also called a lewis structure, a lewis dot diagram, an electron dot structure note the lone pair (dots without bonds) on top of p, just like for n in the previous example for nh3. Lewis structure of carbonate ion is drawn in this tutorial step by step. 7 Lewis Diagrams, Formal Charge ...




















0 Response to "38 use the orbital-filling diagram to show the electron configuration of phosphorus, p."
Post a Comment