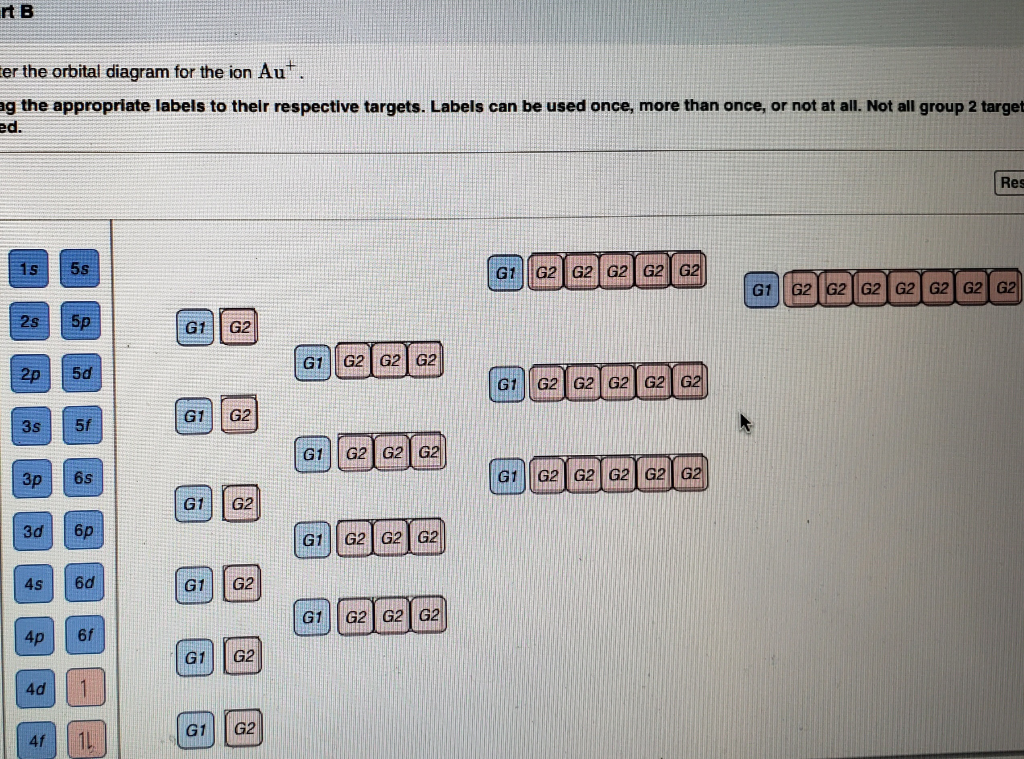
37 orbital diagram for au+
Aug 22, 2014 — An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown .... For example, in the process Au + hν → Au+(L− 1) + *** This review consists of two parts: an overview with marked spoilers and unmarked mild thematic spoilers to help potential readers decide if this work is right for them, and a more in-depth analysis, which contains unmarked moderate spoilers for *Orthogonal*[.](https://upload.wikimedia.org/wikipedia/en/8/88/Orthogonal_%28series%29.jpg) *** #Overview *Tenet*: [Don't try to understand it, feel it.](https://youtu.be/tPEhCcluVdM?t=104) Me: Oh come on, Nolan. That's just lazy, now. You call th...
I'm creating a setting for a sci-fi RPG. I would like to check whether the concept is physically possible. The main action happens on a giant ringworld which centrifugally orbits a paired black hole and star. Here is a diagram of what I mean (not to scale): https://i.stack.imgur.com/27VTm.png I am hoping that this setup would provide a day-night cycle. When the star is visible from a point on the ringworld, it is "day". When the star goes "behind" the black hole, it becomes "night". Constrai...

Orbital diagram for au+
Humanity has never accepted limits. We as a species have always thrived on the impossible. We commanded fire with our primitive minds, walking the line of life and destruction. We conquered mother earth, forcing her to feed us. We reached endlessly for the impossible horizon on the ocean, never once fazed by the fact that no one ever returned. So it was only natural that we refused to acknowledge space as impossible to traverse. Our cloud cities of Venus, our domes across the green Martian valle... Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. a. Cd2+ b. Au+ c. Mo3+ d. Zr2+ Lyniesha W. Numerade Educator View. Problem 79 Which is the larger species in each pair? a. Li or Li+ b. I- or Cs+ c. Cr or Cr3+ d. O or O2-Regina H. ... Hello r/EngineeringStudents Just wondering if it's realistic for the Australian space sector to grow in the foreseeable future as I am looking to make a career in the space sector and moving to the United States doesn't appear to be realistic for me. Some key players are listed below in alphabetical order: [Australian Space Agency](https://en.wikipedia.org/wiki/Australian_Space_Agency) A government body designed to coordinate and regulate commercial spaceflight activities July 2018: F...
Orbital diagram for au+. This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... The first compound in the diagram has two blue and three red atoms. The second compound has two blue and one red atom. Since each compound has two blue atoms we can compare the ratio of red atoms between the Chapter 1. two compounds. Thus, the ratio of red atoms in the two compounds to two blue atoms in the two compounds, is three to one. 1.19 what is the orbital diagram for Au+, how do you fit the f orbitals in? 6,683 results Chemistry. How would the dx2−y2 orbital in the n=5 shell compare to the dx2−y2 orbital in the n=3 subshell? True or False 1.The contour of the orbital would extend further out along the x and y axes. 2. The value of ℓ would increase by 2. 3.The radial Hello, Just wondering if it's realistic for the Australian space sector to grow in the foreseeable future as I am looking to make a career in the space sector Some key players are listed below in alphabetical order: [Australian Space Agency](https://en.wikipedia.org/wiki/Australian_Space_Agency) A government body designed to coordinate and regulate commercial spaceflight activities July 2018: Founding of the Australian Space Agency December 2018: Location is announced at Adelaide, S...
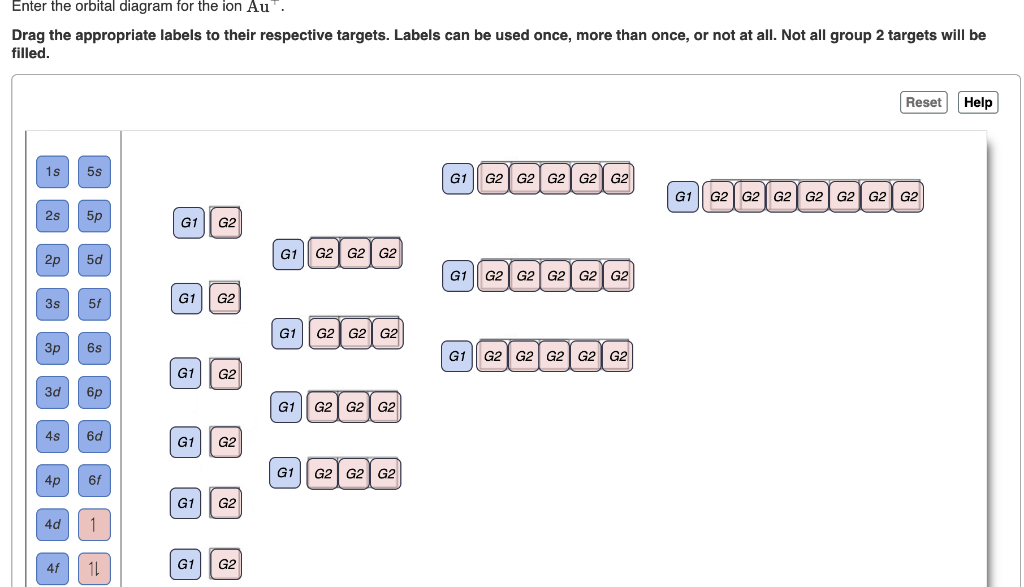
I'm creating a setting for a sci-fi RPG. I would like to check whether the concept is physically possible. The main action happens on a giant ringworld which centrifugally orbits a paired black hole and star. Here is a diagram of what I mean (not to scale): https://i.stack.imgur.com/27VTm.png I am hoping that this setup would provide a day-night cycle. When the star is visible from a point on the ringworld, it is "day". When the star goes "behind" the black hole, it becomes "night". Constrai... This is the continuation of my little series, mainly because I ran out of excuses to not write it. Expect more in the near future. [Dawn of Humanity's Empire: Part 1](https://www.reddit.com/r/HFY/comments/70nxwp/dawn_of_humanitys_empire_part_1/) .............. Five years passed, and humanity's colonies expanded and prospered at unbelievable rates. Admiral Davies remained our ambassador to the other civilizations, conducting first contact with twenty individual species. In his many travels he ... With a few simple calculations, it looks like ISON might be a naked eye object on Mars later this year. Using the [JPL HORIZONS](http://ssd.jpl.nasa.gov/horizons.cgi) ephemerides for Mars and ISON, they'll be closest at about 14:00 UT on 1 Oct 2013. We can extract the Earth-Mars distance (2.13351 AU), the Earth-ISON distance (2.13317 AU), and the ISON apparent visual magnitude as seen from Earth (10.96; though note that the [IAU Minor Planet Center](http://scully.cfa.harvard.edu/cgi-bin/returnp... Enter the orbital diagram for the ion Au+ ‣ When an element is a cation (+) you REMOVE electrons. ‣ Electrons are generally removed from the "s" sub-level 1.) Remove one electron from 5s1 ANSWER: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10.
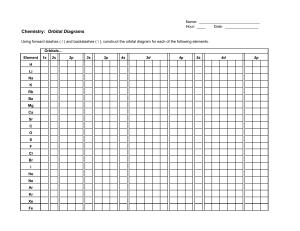
68.Write orbital diagrams for each ion and indicate whether the ion is diamagnetic or paramagnetic. a. Cd2 + b. Au+ c. Mo3 + d. Zr2 + Ionic Electron Configurations, Ionic Radii, Magnetic Properties, and Ionization Energy 70. Which is the larger species in each pair? a. Sr or Sr2 + b. Newton class starship MSCV *Empiricist* Circular Orbit 15 AU from Luyten’s Star January 2219 For whatever reason, wake-up alarms had always been designed by sadists. That was, at least, the only plausible explanation that came to the mind of Mission Commander Ivy Czininski as a horrible spike of sound drilled down into a rather pleasant dream about her vacation on Earth, and ripped her back into reality. A reality where, when she examined the time, she found to her disgust that she had sle... How would the dx2−y2 orbital in the n=5 shell compare to the dx2−y2 orbital in the n=3 subshell? True or False 1.The contour of the orbital would extend further out along the x and y axes. 2. The value of ℓ would increase by . chemistry. Build the orbital diagram for the ion most likely formed by phosphorus. chemistry Orbital Diagram of Zinc (Zn), Electron Configuration, and . ... its For Au+, one electron is removed from the outermost 6s orbital, making the configuration .... May 5, 2014 — SOLUTION: PROBLEM: Name the Period 3 element with the following ionization energies.
Sodium chloride is added slowly to a solution that is 0.010 M in Cu+, Ag+, and Au+. The Ksp values for the chloride salts are 1.9 ´ 10-7, 1.6 ´ 10-10, and 2.0 ´ 10-13, respectively. Which compound will precipitate first?
>I'm keeping the original post in tact, but be sure to check the edits! Some changes are being made... > >Also! Thank you so much for the silver et al, and for all the great advise! A lot of people are explaining that we had plastics in antiquity if you consider anything malleable ("plastic") or anything with polymers to be plastic. Thanks for the learning opportunity! But to be clear, I intended to mean oil-based plastic, like (in this case) polyethylene: what our plastic bags and ...
## r/worldnews - [DueyDerp](https://reddit.com/u/DueyDerp) **One arrest leads to discovery of global online paedophile network** [Comments](https://reddit.com/r/worldnews/comments/jrs2mk/one_arrest_leads_to_discovery_of_global_online/) || [Link](https://www.smh.com.au/national/nsw/global-online-paedophile-photo-and-video-network-allegedly-uncovered-after-nsw-child-sex-arrest-20201110-p56d6z.html) - [fruitspunch-samuraiG](https://reddit.com/u/fruitspunch-samuraiG) **Jair Bolsonaro asks Brazi...
A step-by-step description of how to write the electron configuration for Gold (Au). In order to write the Au electron configuration we first need to know t...
(Northern Arizona University) and Raymond Chang, this success guide is written for use with General Chemistry. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems.
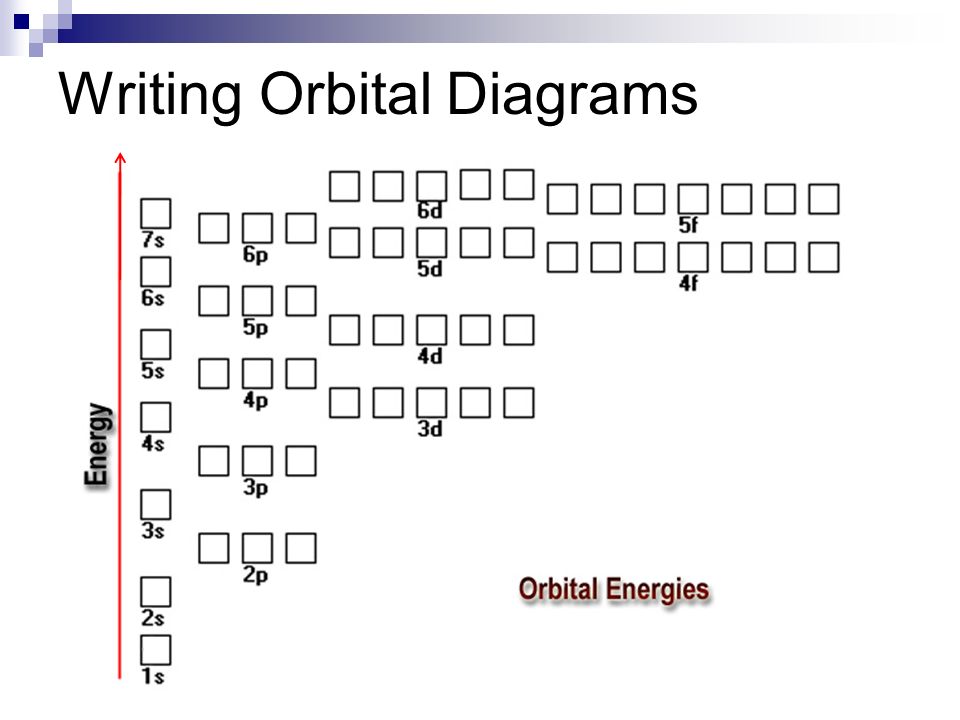
So for scandium the 1st and 2nd electron must be in 1s orbital, the 3rd and 4th in the 2s, the 5th through 10th in the 2p orbitals, etc. You are watching: Enter the orbital diagram for the ion au+. This is a memory device to remember the order of orbitals for the first two quantum numbers. Follow the arrow starting in the upper right, when the ...
Since Au+ has lost one electron and the numbers (or arrows in the diagram) represent the electrons, its configuration is: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10. So there will not be the 6s orbital.
The following is mostly taken from "A Little Book of Coincidence in the Solar System" by John Martineau. I've attempted to summarize some of the coincidences described in this book. While doing so I came across even more coincidences to add to this burgeoning list. >There may be fundamental relationships between space, time and life which have not yet been understood. >Is it all just a coincidence, or do the patterns perhaps explain the scientists? ##The Seven There are seven clearly...
... as [originally posted](http://www.planetary.org/blogs/emily-lakdawalla/2016/10041634-whats-up-october-2016.html) by Emily Lakdawalla on the Planetary Society blog ( [see accompanying diagram](http://www.planetary.org/multimedia/space-images/charts/whats-up-in-the-solar-system-frohn.html) ) ... relevant subreddits are **emphasized** here (such links aren't in her original post) ... her post itself has a plethora of fantastic links, pictures, and video clips. Please go check it out! --------...
Here's a collection of WoB's from last month. Discuss in the comments, and if we missed one that you found interesting feel free to share it! Help us collect WoB's for next month [here](/r/cosmere/wiki/wob). --- ####Sel [The earthquake that caused the Reod is not specifically related to the ground’s sentience](http://www.17thshard.com/forum/topic/58194-new-wob/#comment-538765) [Shai might show up in other stories. (no Emperor's Soul sequels)](/r/Fantasy/comments/5t9nyy/-/ddo6wjj/?context=...
This means removing electrons right to left from your neutral atom configuration. In order to get your correct neutral atom configuration, once you have the orbitals and the electrons in them, you must order them from lowest to highest energy. Then you remove 3 electrons to give you Tl+3: [Xe] 4f14 5d10.
Hey, /r/astronomy! I recently answered a [question](https://www.reddit.com/r/askscience/comments/3esx20/how_does_pluto_sustain_any_kind_of_atmosphere/) on /r/AskScience about how Pluto can sustain an atmosphere while the Moon does not. This got me thinking that perhaps people would like to know more about atmospheres as an astronomical phenomenon in general. As such I have decided to write a post which will talk about atmospheres of stars and planets in general as well as the atmospheres in our ...
Hello [r/AskEngineers](https://www.reddit.com/r/AskEngineers/), Just wondering if it's realistic for the Australian space sector to grow in the foreseeable future as I am looking to make a career in the space sector and moving to the United States doesn't appear to be realistic for me. Some key players are listed below in alphabetical order: [Australian Space Agency](https://en.wikipedia.org/wiki/Australian_Space_Agency) A government body designed to coordinate and regulate commercial spa...
[Xe] 4f^14 5d^10 The atomic number of Au is 79. Therefore, its configuration is: 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^6 4d^10 5s^2 5p^6 4f^14 5d^10 6s^1 or, [Xe] 4f^14 5d^10 6s^1 For Au^+, one electron is removed from the outermost 6s orbital, making the configuration, [Xe] 4f^14 5d^10
Doesn't the 5s come before the 4d? I chose [Kr]5s^2 on a quiz but it was wrong. Therefore, the ground state electron configuration for Zr 2+ is : [Kr]4d 2 5s 2. In^+1 [Kr] 5s2 4d10. 1s2, 2s2, 2p6. 1) nuclear charge and relative energy of 3d and 4s orbitals, 2) relative e-e repulsions in 3d and 4s orbitals, 3) exchange energy. Booster Classes. Need an editable periodic table to edit? Enter the ...
Each orbital group must fill before moving to the next orbital group. 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p . Germanium #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^2. Germainum is in the 4th row Energy Level of the periodic table. The element is in the 2nd column of the p block, Group IVA (Column 13).
Academia.edu is a platform for academics to share research papers.
Figure A periodic table of partial ground- state electron configurations. Figure Write orbital diagram for Au+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy. Click within the orbital to add electrons. Jul 21, · Write orbital diagram for Au+ Determine if the ion is diamagnetic or ...
A) Write orbital diagram for Au+. B) Write orbital diagram for Zr2+. Expert Answer. What is the electron configuration of Au+? Get this answer with Chegg Study View this answer. Previous question Next question. Need an extra hand? Browse hundreds of Chemistry tutors. Nov 23, · which orbitals in the molecular orbital diagram contain the lone ...
**[Part 1](https://old.reddit.com/r/NateLundberg/comments/i022g8/my_father_taught_me_how_to_look_at_the_night_sky/)** || **[Part 2](https://old.reddit.com/r/NateLundberg/comments/i0u58m/my_father_taught_me_how_to_look_at_the_night_sky/)** || **[Part 3](https://old.reddit.com/r/NateLundberg/comments/i3073i/my_father_taught_me_how_to_look_at_the_night_sky/)** || **[Part 4](https://old.reddit.com/r/NateLundberg/comments/i97r6f/my_father_taught_me_how_to_look_at_the_night_sky/)** “Will, Kristen, do...
Answer to: Draw the orbital diagram for Au+. By signing up, you'll get thousands of step-by-step solutions to your homework questions. You can also...
Orbital diagram of Nitrogen (N) 8. Orbital diagram of Oxygen (O) 9. Orbital diagram of Fluorine (F) 10. Orbital diagram of Neon (Ne) 11. Orbital diagram of Sodium (Na)
The following is mostly taken from "A Little Book of Coincidence in the Solar System" by John Martineau. I've attempted to summarize some of the coincidences described in this book. While doing so I came across even more coincidences to add to this burgeoning list. >There may be fundamental relationships between space, time and life which have not yet been understood. >Is it all just a coincidence, or do the patterns perhaps explain the scientists? ##The Seven There are seven clearly...
An orbital diagram is the representation of electrons in the orbitals. The symbol of gold is Au. It is a transition metal with atomic number 79. We use this concept of the orbital diagram for ...
Electrons orbit the nucleus in energy levels, which are also called shells. 4p2. In metals, and in many other solids, the atoms are arranged in regular arrays called crystals. 3p6. 86% (221 ratings) Problem Details. The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2.
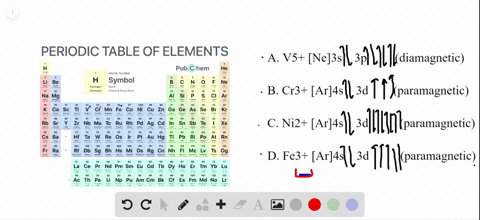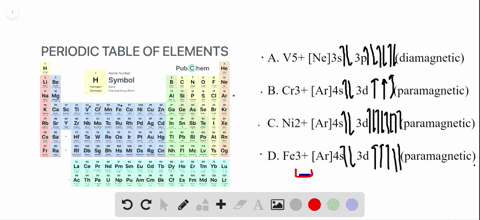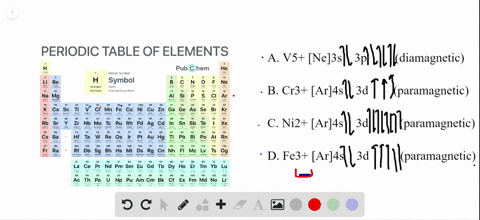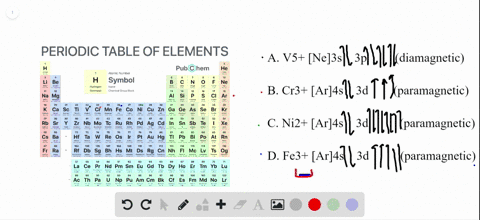
The orbital diagram for Au + is: Since there are 2 unpaired electrons, Au + is paramagnetic. 85% (16 ratings) Problem Details. Identify whether the ions are diamagnetic or paramagnetic. a. Cd 2+ b. Au + c. Mo 3+ d. Zr 2+ Learn this topic by watching Paramagnetism and Diamagnetism Concept Videos.
**ARRIVING SHORTLY.** The signal blanketed the Northern Hemisphere for 24 hours. Repeated in 10 languages, in morse and ASCII. Initially panic, then a widespread assumption of prankery. Less suicides than you'd think. Earth's median feeling was giddy uncertainty. A movie deal was made. A week later, an amateur astronomer noticed an anomaly above Mercury. "Big Shiny Thing". His words. Radio signals were sent. No response. The Hubble was pointed at it. 100 km across. Changes on Mercury's Surface...
Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11.
Answer to Write orbital diagram for Mo3+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing.write orbital diagram for each ion and determine if the ion is diamagnetic or paramagnetic. a. Cd 2+ diagramweb.net + diagramweb.net 3+ d. Zr 2+ Provide your answer: example:paramagnetic, diamagnetic, etc., .
3.5 Mm To Xlr Wiring Diagram; Kohler Cv490s Wiring Diagram; Pioneer Avh 270bt Wiring Diagram; Orbital Diagram For Au+; Maytag Ldg7500aaw Wiring Diagram; Westerbeke Generator Parts Diagram; Wiring Diagram For Simplicity Sunstar 1691528; Gm 10si Alternator Wiring; Avital Remote Starter Wiring Diagram; Wiring Diagram Pioneer Dxh6800
The Oort Cloud description: >The Oort cloud (/ɔːrt, ʊərt/), sometimes called the Öpik–Oort cloud, first described in 1950 by Dutch astronomer Jan Oort, is a theoretical concept of a cloud of predominantly icy planetesimals proposed to surround the Sun at distances ranging from 2,000 to 200,000 au (0.03 to 3.2 light-years). Wikipedia [Here's a diagram](https://upload.wikimedia.org/wikipedia/commons/thumb/d/da/PIA17046_-_Voyager_1_Goes_Interstellar.jpg/800px-PIA17046_-_Voyager_1_Goes_Interst...
**Betiane Sainte-Victorie** *"Mankind will die one day, the moment we stop being curious."* **Betiane Sainte-Victorie** (*Betiane Sen-Viktwa* in Haitian Creole) was a Haitian physicist and engineer, and widely credited for both the pioneering of the field of FTL travel among humanity, along with building the first prototypes, by hand, in Port-au-Prince. Because of this, she had been popularized as the Mother of Hyperdrive. Born to a poor family during the UN invasion of the Caribbean, Sainte-...
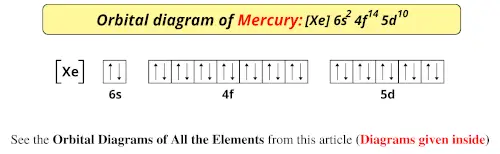
The orbital diagram for gold starts with the base [Xe], which is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6. The outer shells are 6s2 5d9.
Answer to Write orbital diagram for Au+. Determine if the ion is diamagnetic or paramagnetic. The atomic number of Au is Therefore, its For Au+, one electron is removed from the outermost 6s orbital, making the configuration. orbital diagram for Au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1 you just have to fill the "boxes ...
(Author's note: First part here: https://www.reddit.com/r/HFY/comments/3g6n3g/oc_budgetary_meeting/) **Emergency Meeting of Xenology Subcommittee, Science Directorate Central Council. 44404.1.37 Standard Galactic Reckoning** Subject: Special request for emergency funding Called to order by Council Secretary Jin Secretary Jin was unhappy, a fact he made evident to each member of the subcommittee by making impatient gestures as they filed in. Unlike councilor Pafh, who had spent the last five...
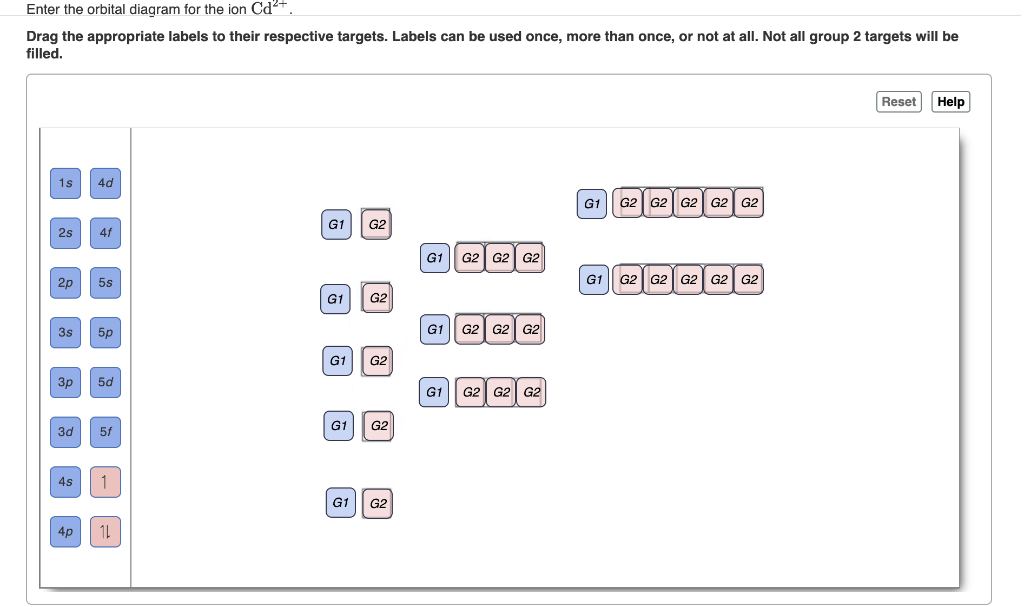
Chemistry questions and answers. Enter the orbital diagram for the ion Cd2+ Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all group 2 targets will be filled. Enter the orbital diagram for the ion Au+. Drag the appropriate labels to their respective targets.
Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. a. Cd2+ b. Au+ c. Mo3+ d. Zr2+











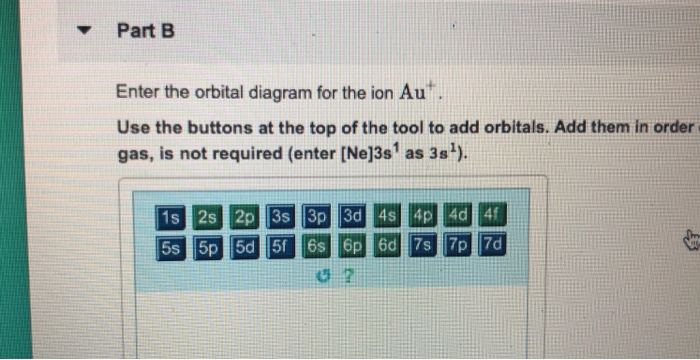
0 Response to "37 orbital diagram for au+"
Post a Comment