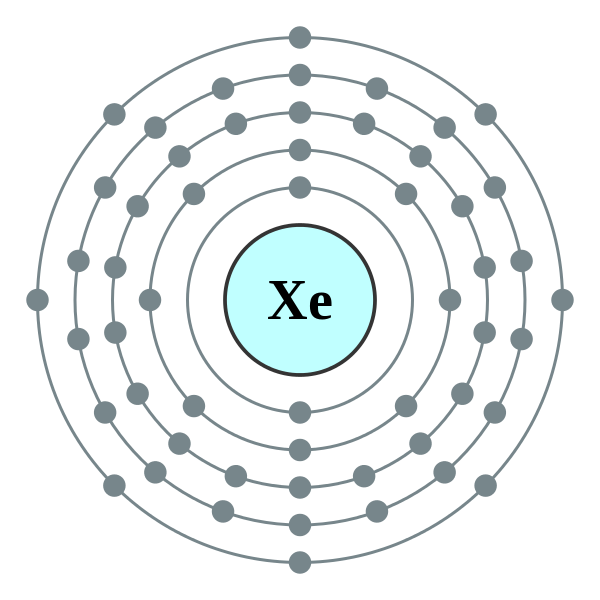
37 electron dot diagram for xenon
June 4, 2018 - Electron dot diagrams, sometimes called Lewis dot diagrams, were first used by Gilbert N. Lewis in 1916. These diagrams are used as a shorthand notation to show the number of valence electrons in an atom.
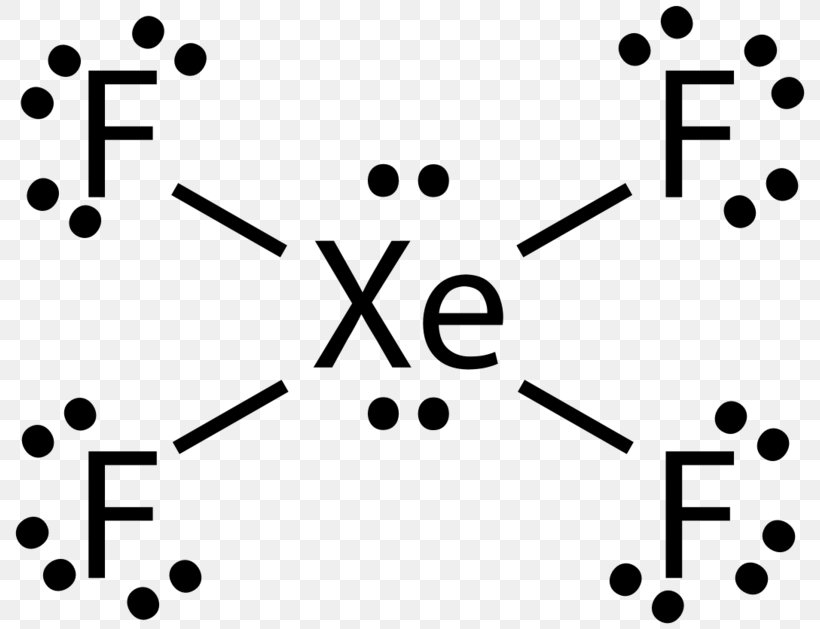
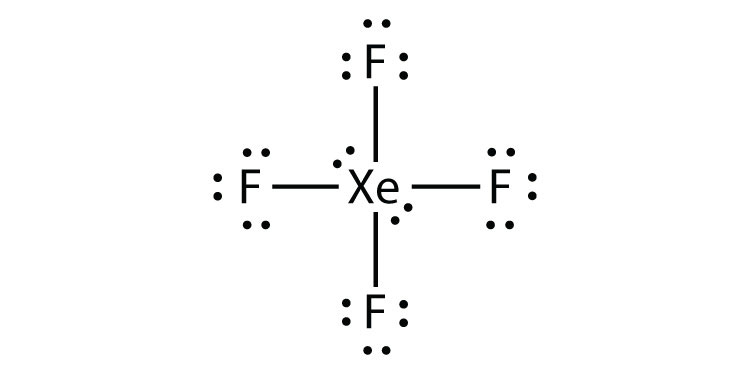
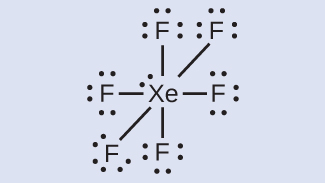
It has two lone pairs of nonbonding electrons on the central atom of Xenon. The molecule has octahedral electron geometry and square planar molecular geometry. XeF4 is a nonpolar molecule and has sp3d2 hybridization. At the Geometry of Molecules, we like knowing what you think. So let us know your thoughts on this molecule in the comments below.
August 23, 2017 - Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.

Electron dot diagram for xenon
June 16, 2012 - Gilbert N. Lewis is widely known for his use of simple symbolic representations of elements that show valence electrons as dots. You've seen what the are Bohr diagrams for the first 20 elements. Sometimes it is more convenient to represent the elements by its Lewis electron-dot symbol.
What is the electron dot diagram for xenon? - Answers The electron dot, or Lewis dot diagram for xenon is the symbol Xe surrounded by four pairs of dots, representing eight valence Refer to the...
See the Big List of Lewis Structures · About this Site | Report a Problem | Comments & Suggestions
Electron dot diagram for xenon.
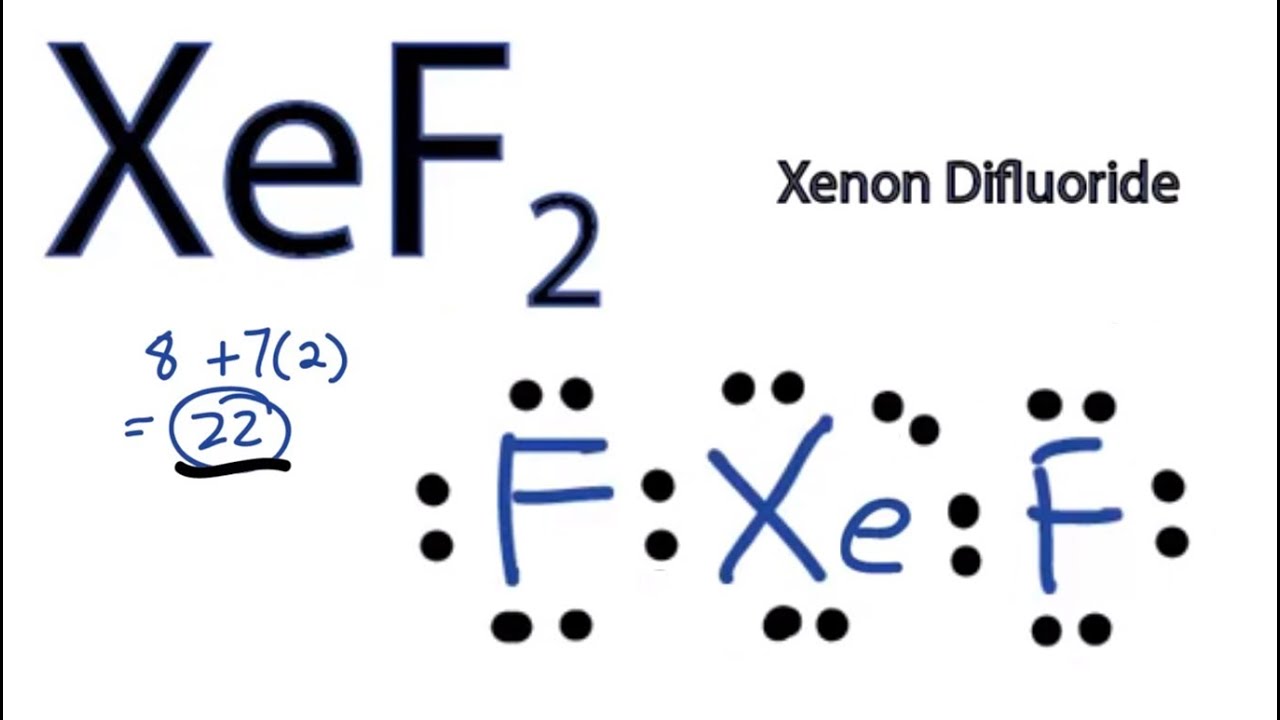
Lewis Structures for XeF2. Step-by-step tutorial for drawing the Lewis Structure for XeF2.
A Lewis electron dot diagramA representation of the valence electrons of an atom that uses dots around the symbol of the element. (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element.
Xenon is a group IA element and has 8 electrons in its last shell (valence shell). Fluorine is a group VIIA element in the periodic table and contains 7 electrons in their last shell. Now we know how many electrons includes in valence shells of xenon and fluorine atom. valence electrons given by fluorine atoms = 7 * 4 = 28
A step-by-step explanation of how to draw the XeF2 Lewis Dot Structure (Xenon difluroide).For the XeF2 structure use the periodic table to find the total num...
A step-by-step explanation of how to draw the Xe Lewis Dot Structure.For the Xe structure use the periodic table to find the total number of valence electron...
How to Draw a Lewis Structure for XeO3 xenon trioxide?Lewis Structure: https://www.youtube.com/watch?v=4rRVPeeZRmc&list=PLDwv-O7TJyNjAB0ak6We0sQ8t_a7D2cJ7Sub...
August 22, 2021 - For the same reason six or seven bonds are possible and xenon can form eight covalent bonds in the XeO4 compound! Food elements may have more than eight valence electrons, but they do not tend to form covalent bonds. ... A Lewis electronic dot diagram (or electronic dot diagram or Lewis diagram ...
August 15, 2020 - Three cases can be constructed that do not follow the Octet Rule, and as such, they are known as the exceptions to the Octet Rule. Following the Octet Rule for Lewis Dot Structures leads to the most …
Comprehensive data on the chemical element Xenon is provided on this page; including scores of properties, element names in many languages, most known nuclides of Xenon. Common chemical compounds are also provided for many elements. In addition technical terms are linked to their definitions ...
For xenon atom ⇒ Valence electrons of xenon = 8 ⇒ Lone pair electrons on xenon = 0 ⇒ Bonding electrons around xenon (4 single bonds) = 8 ∴ (8 – 0 – 8/2) = +4 formal charge on the xenon central atom. The above XeO4 lewis structure is not stable because of the high formal charge.
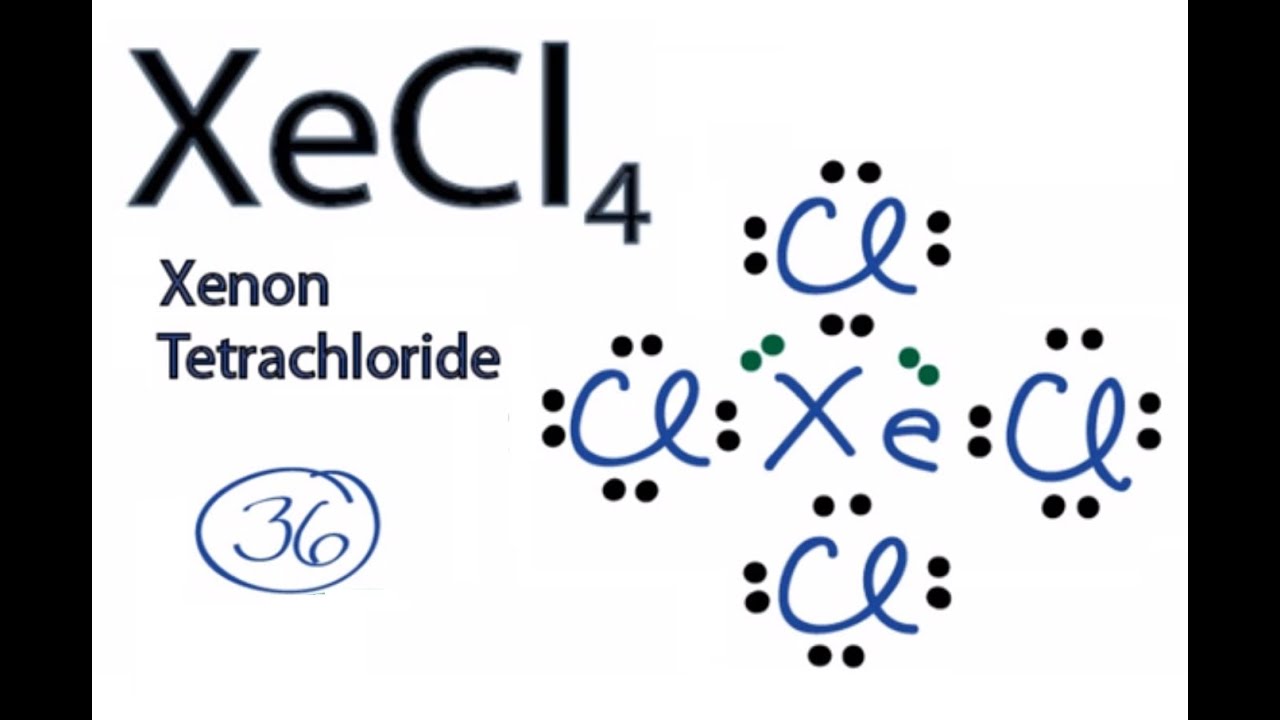
A step-by-step explanation of how to draw the XeBr4 Lewis Dot Structure.For the XeBr4 structure use the periodic table to find the total number of valence el...
When we write the Lewis structures for these molecules, we find that we have electrons left over after filling the valence shells of the outer atoms with eight electrons. These additional electrons must be assigned to the central atom. ... Writing Lewis Structures: Octet Rule Violations Xenon is ...
Dr. McCord's online textbook resource for CH301N and CH302N
In some molecules, the central atom exceeds the octet rule (is surrounded by more than eight electrons). See an example of a molecule that violates the octet rule (XeF₂) and learn how to draw its Lewis diagram in this video.
Full electron configuration of xenon: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 · iodine ← xenon → cesium
The electron dot, or Lewis dot diagram for xenon is the symbol Xe surrounded by four pairs of dots, representing eight valence electrons. Refer to the related link for an illu. The Lewis structure for XeF4 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule. Remember that Xenon can .
1 Feb 2021 — You can here understand the Xe valence electrons representation. The dot diagram represents the numbers of Xenon valence electrons.
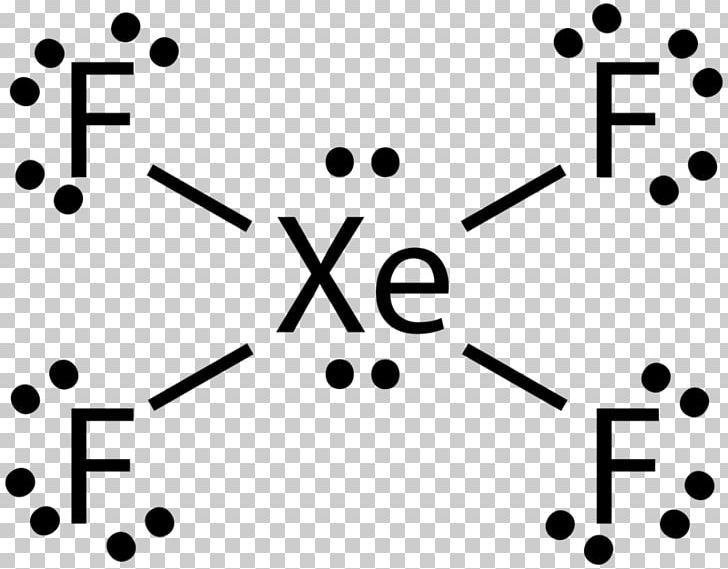
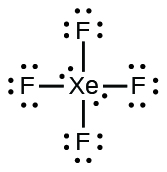
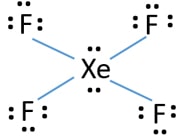
Xenon has 8 dots (4 pairs of dots) around the letters Xe. for XeF4. Step-by-step tutorial for drawing the Lewis Structure for XeF4. for the molecule. Remember that Xenon can have more than 8 valence electrons.
Lewis Dot of Xenon Tetrafluoride · Back70 More Lewis Dot StructuresXe does not follow the octet rule. It actually bonds. It will hold more than 8 electrons. Xenon having valence electrons in the 4th energy level, will also have access to the 4d sublevel, thus allowing for more than 8 electrons.
August 31, 2021 - Lewis structures, also known as ... or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. ... Why are they so expensive? Xenon HID bulbs are more expensive ...
August 15, 2020 - Lewis Symbols are simplified Bohr diagrams which only display electrons in the outermost energy level. The omitted electrons are those in filled energy levels, which do not contribute to the chemical …
Xenon (Xe) has an atomic mass of 54. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
October 12, 2015 - The reason why Xenon can bond to 6 Fluorines is because it is an exception to the octet rule. Some elements in period 3 or high can hold more than an octet due to the 3d orbitals that are available for electrons to be added. These elements that are exceptions can have up to 10, 12, or 14 electrons ...
March 24, 2021 - Lewis dot symbols provide a simple rationalization of why elements form compounds with the observed stoichiometries. A plot of the overall energy of a covalent bond as a function of internuclear …
Lewis Structures for XeF4. Step-by-step tutorial for drawing the Lewis Structure for XeF4.








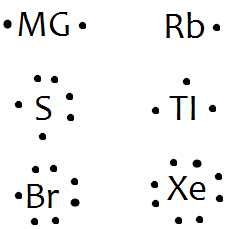

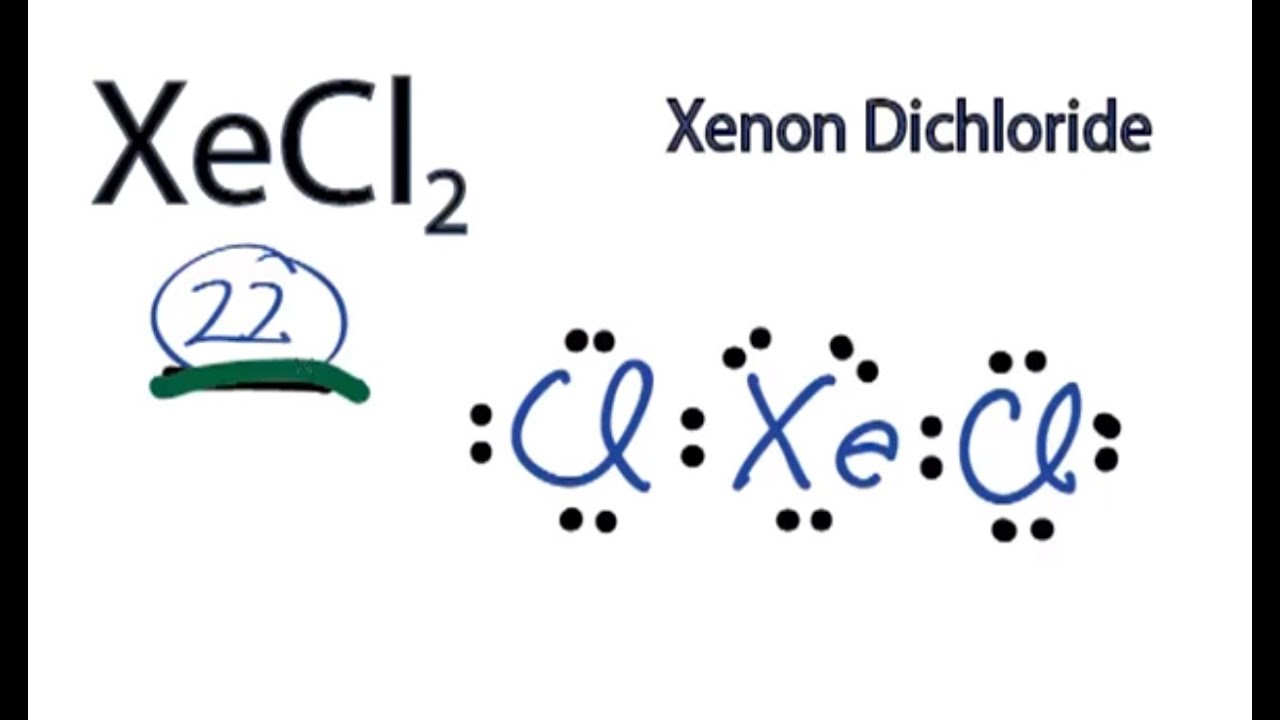
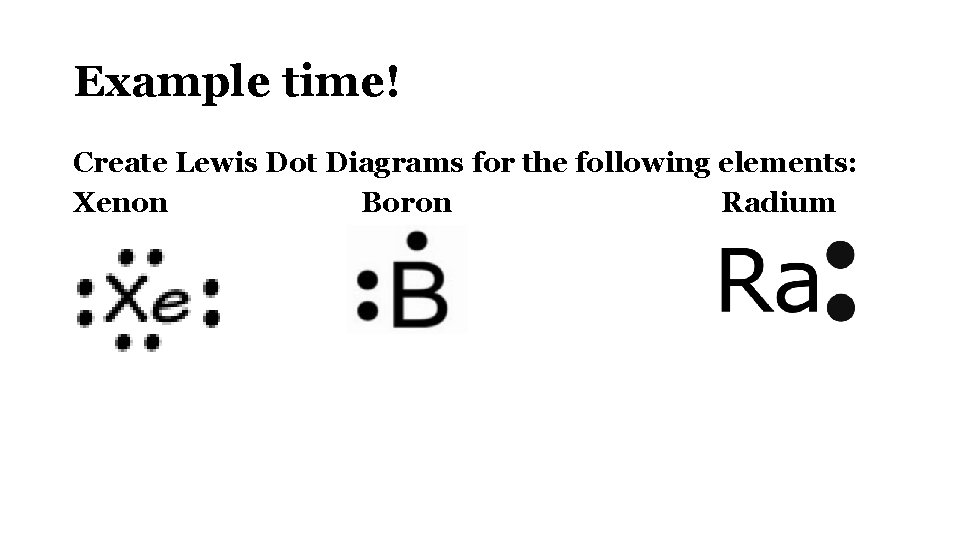










0 Response to "37 electron dot diagram for xenon"
Post a Comment