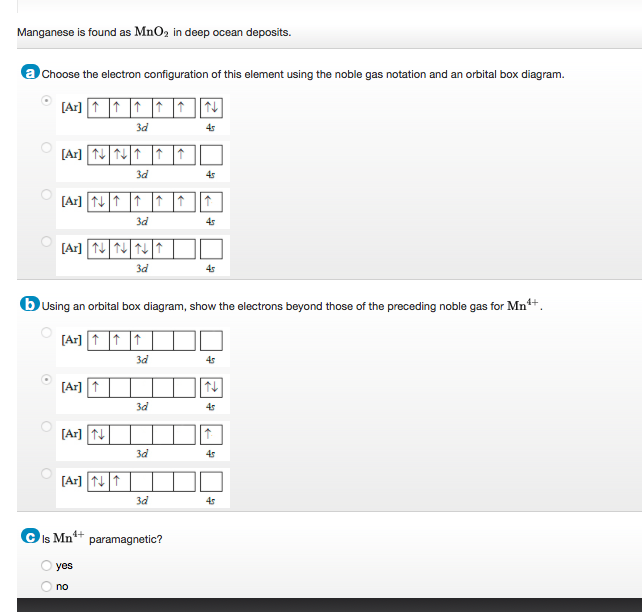
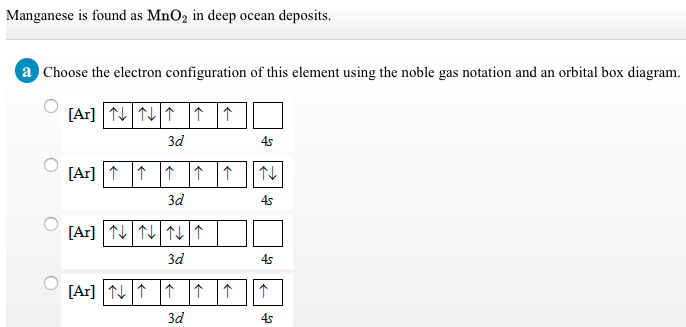
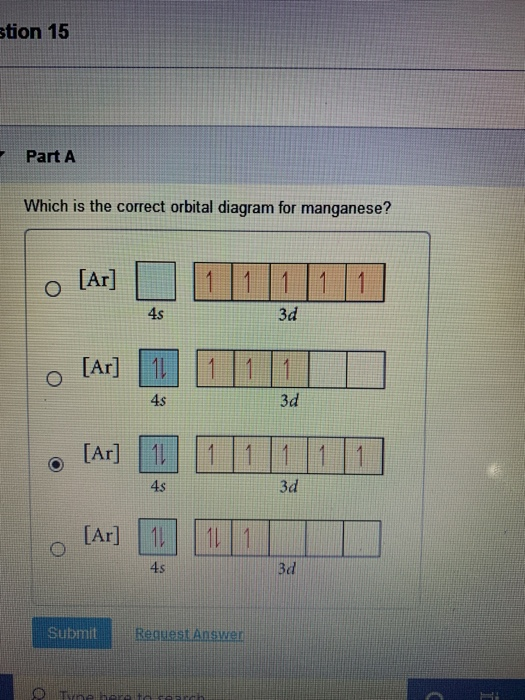
37 choose the correct orbital diagram for manganese.
Review I Constants / Choose the correct orbital diagram for manganese, O [Ar] 11 1 1 3d 45 [Ar] 11 111 4s 3d [Ar] 11 1 1 1 1 1 3d 4s [Ar] 11 111 3d ; Question: Review I Constants / Choose the correct orbital diagram for manganese, O [Ar] 11 1 1 3d 45 [Ar] 11 111 4s 3d [Ar] 11 1 1 1 1 1 3d 4s [Ar] 11 111 3d . This problem has been solved!
Choose the one you consider correct and record your choice in soft pencil on the separate Answer Sheet. Read the instructions on the Answer Sheet very carefully. Each correct answer will score one mark. A mark will not be deducted for a wrong answer. Any rough working should be done in this booklet. Electronic calculators may be used.
There are a few rules for the box and arrow configurations. Aufbau Principle - electrons fill orbitals starting at the lowest available energy state before filling higher states (1s before 2s).. Pauli Exclusion Principle. An orbital can hold 0, 1, or 2 electrons only, and if there are two electrons in the orbital, they must have opposite (paired) spins.
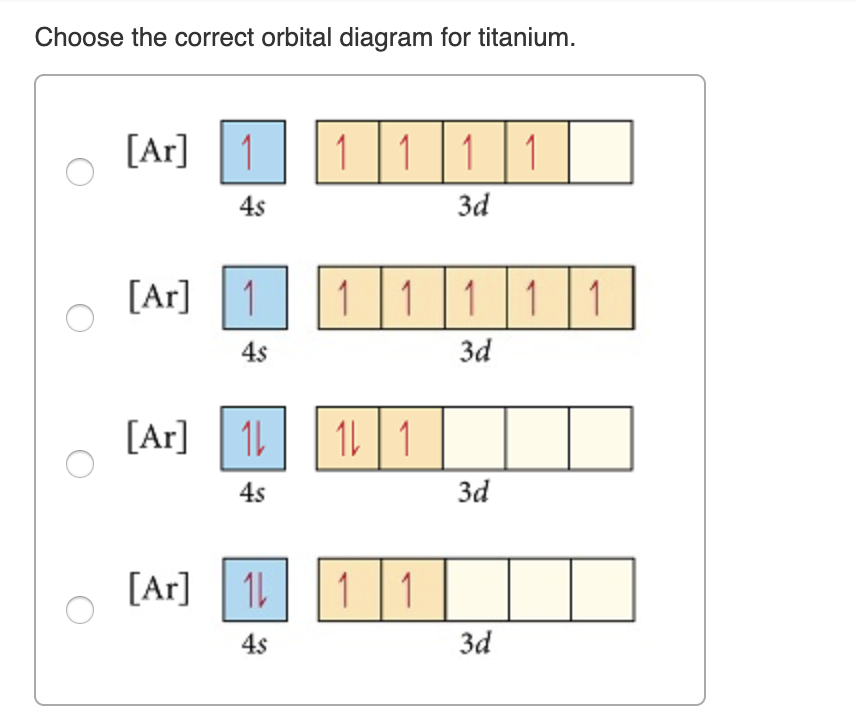
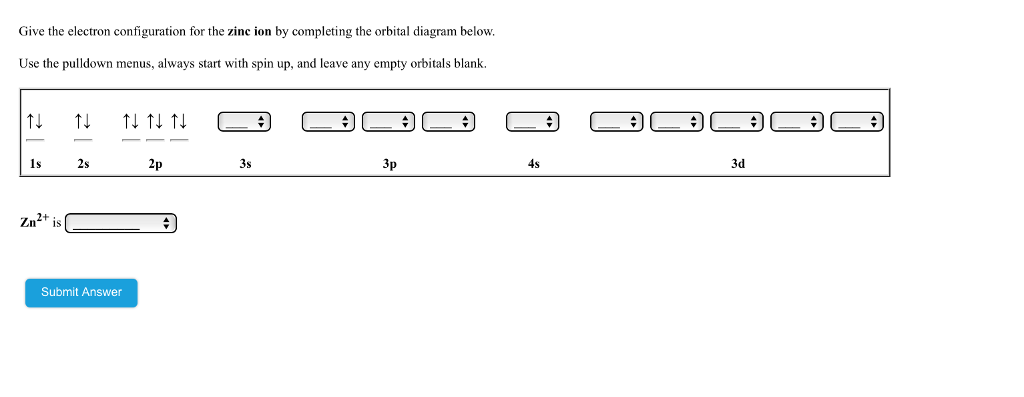
Choose the correct orbital diagram for manganese.
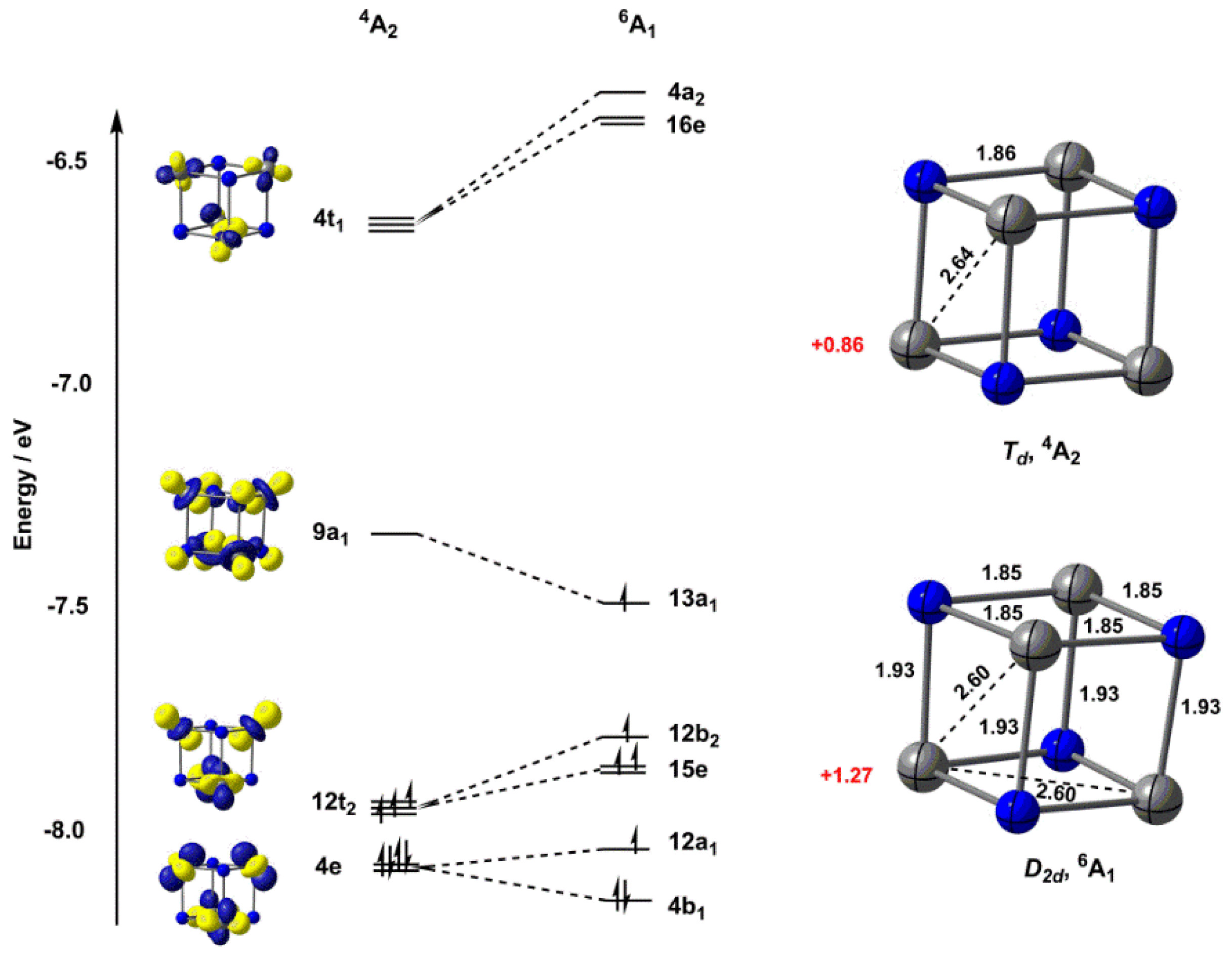
Crystal field theory was developed by considering two compounds: manganese (II) oxide, MnO, and copper (I) chloride, CuCl. Octahedral Crystal Fields. Each Mn 2+ ion in manganese (II) oxide is surrounded by six O 2- ions arranged toward the corners of an octahedron, as shown in the figure below. MnO is therefore a model for an octahedral complex ...
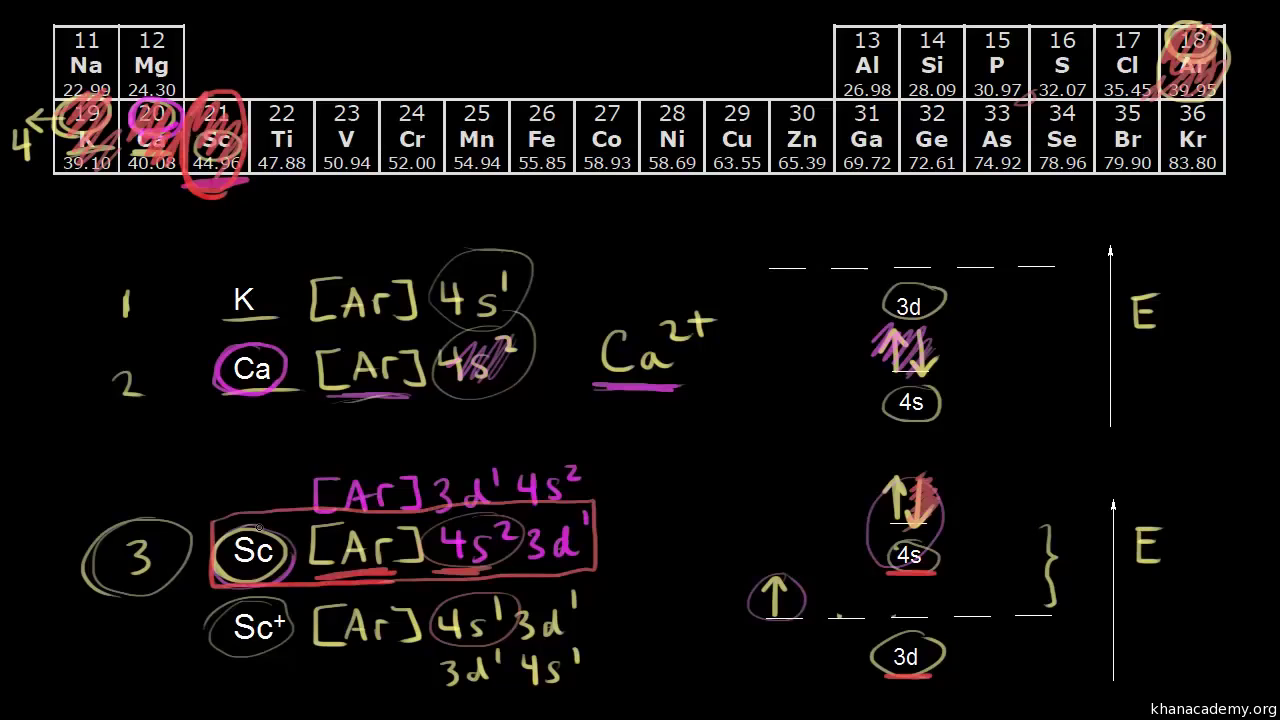
The orbital diagram, the electron configuration and the energy diagram. All three ways are useful. The next atom is helium with 2 electrons. So the second electron could go into the 1s orbital with the opposite spin of the first electron or it could go into the next orbital in the n = 2 level.
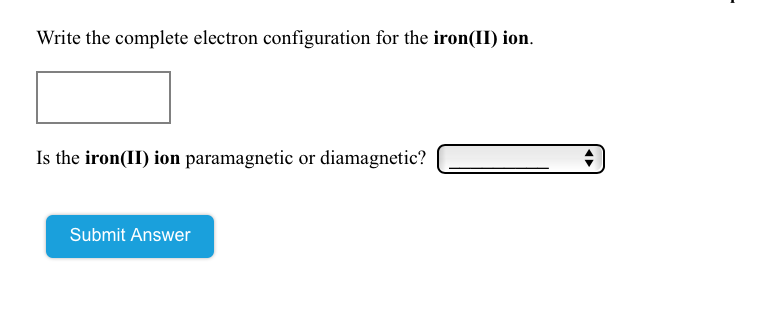
After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d6. Therefore the Iron electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Note that when writing the electron configuration for an atom like Fe, the 3d is usually written before the 4s. Both of the configurations have the correct numbers of ...
Choose the correct orbital diagram for manganese..
Oganesson (element 118 is a good example to show the order of the orbitals. Its electron configuration is: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2 5f 14 6d 10 7p 6. Alternatively, write the symbol for the noble gas before an element (radon, in this case), and just add the extra information:
Which of the following is the correct electron configuration for manganese? answer choices . 1s2 2s2 2p6 3s2 3d6 4s2 3p5. 1s2 2s2 2p6 3s2 3p5 4s2 3p6 ... Choose the correct Lewis Dot diagram for NaCl. answer choices . Tags: Question 16 . ... which of the above orbital diagrams is the best representation of electrons in an unexcited atom?
Choose the orbital diagram that represents the ground state of oxygen. ... The ground-state electronic configuration of the manganese atom, Mn, is(A) 1s22s22p63s23p64s24d5(B) 1s22s22p63s23p63d7(C) 1s22s22p63s23p64s24p5(D) 1s22s22p63s23p63d54s2 ... Ask unlimited questions and get expert help right away.
Orbital Diagram. 1s ... The steel in railroad tracks can contain as much as 1.2% manganese. It is crucial to the effectiveness of vitamin B1. Sources Most abundant ores are pyrolusite (MnO2), psilomelane [(Ba,H2O)2Mn5O10] and rhodochrosite (MnCO3). Pure metal produced by mixing MnO2 with powered Al and ignited in a furnace.
Which of the following is the correct orbital diagram for a nitrogen (n) atom_. Write out the orbital box diagram and the condensed electron configuration for Silicon: 14si=1s^2, 2s^2, 2p^6, 3s^2, 3p^2 / condensed - E:C: = [Ne] 3s^2, 3p^2 9. The two dots represent the n nuclei.
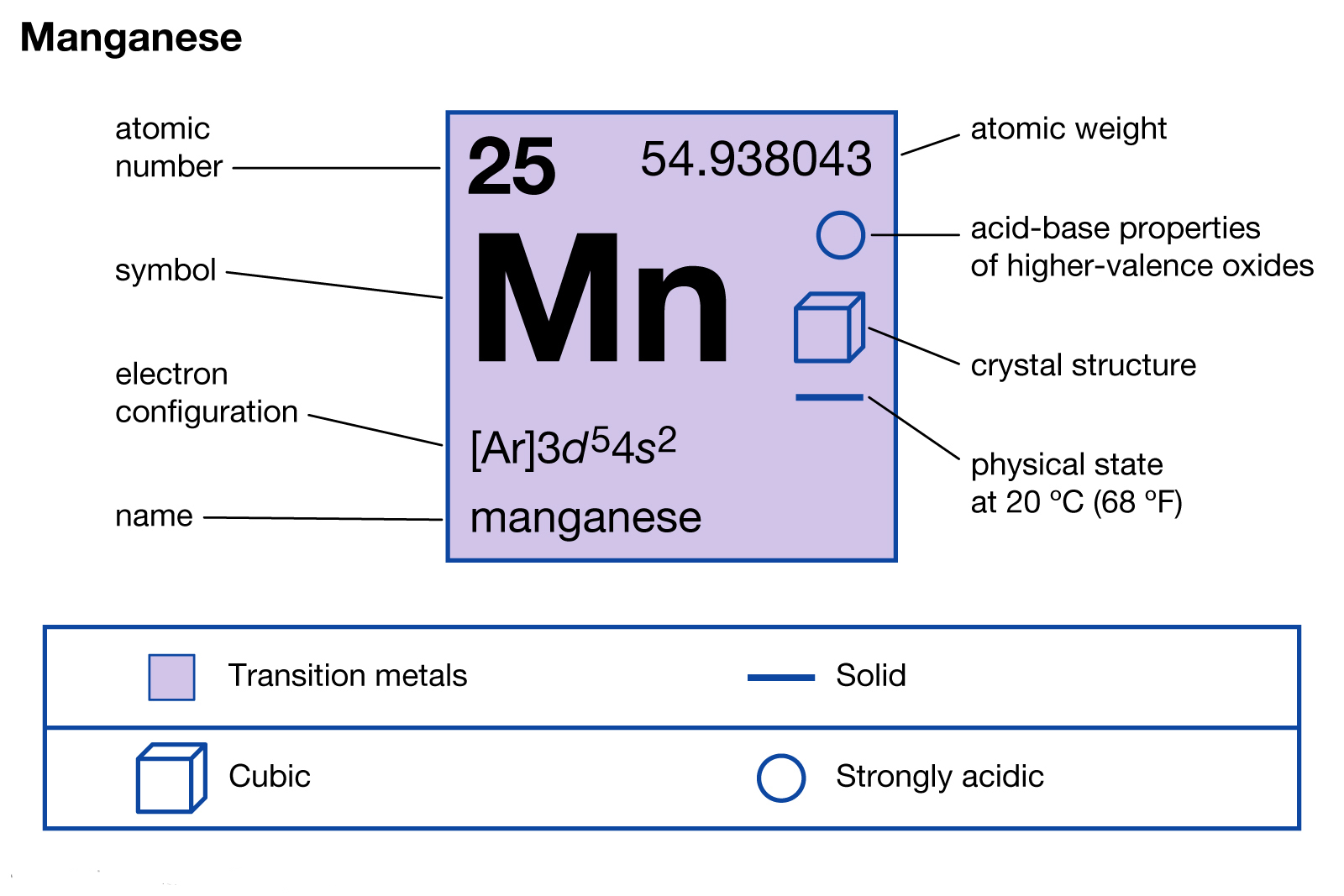
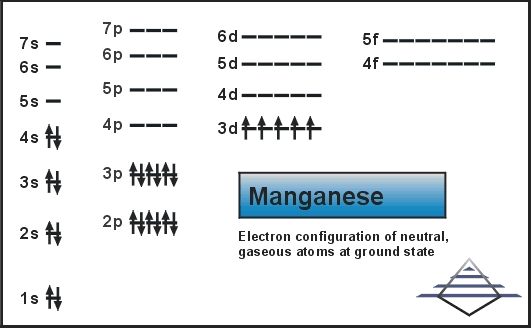
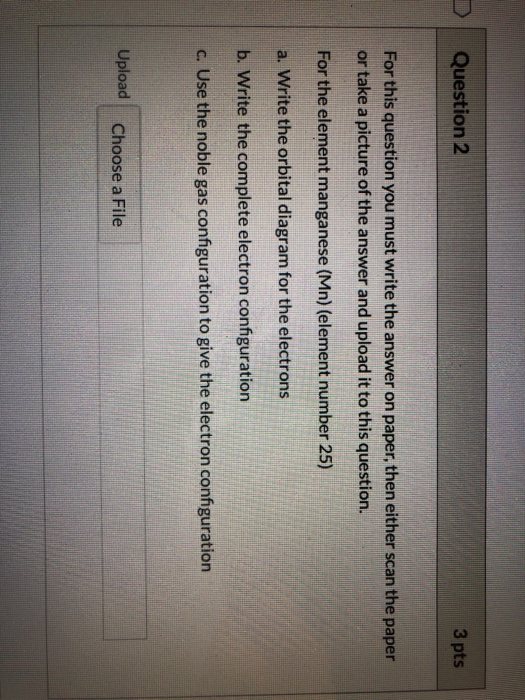
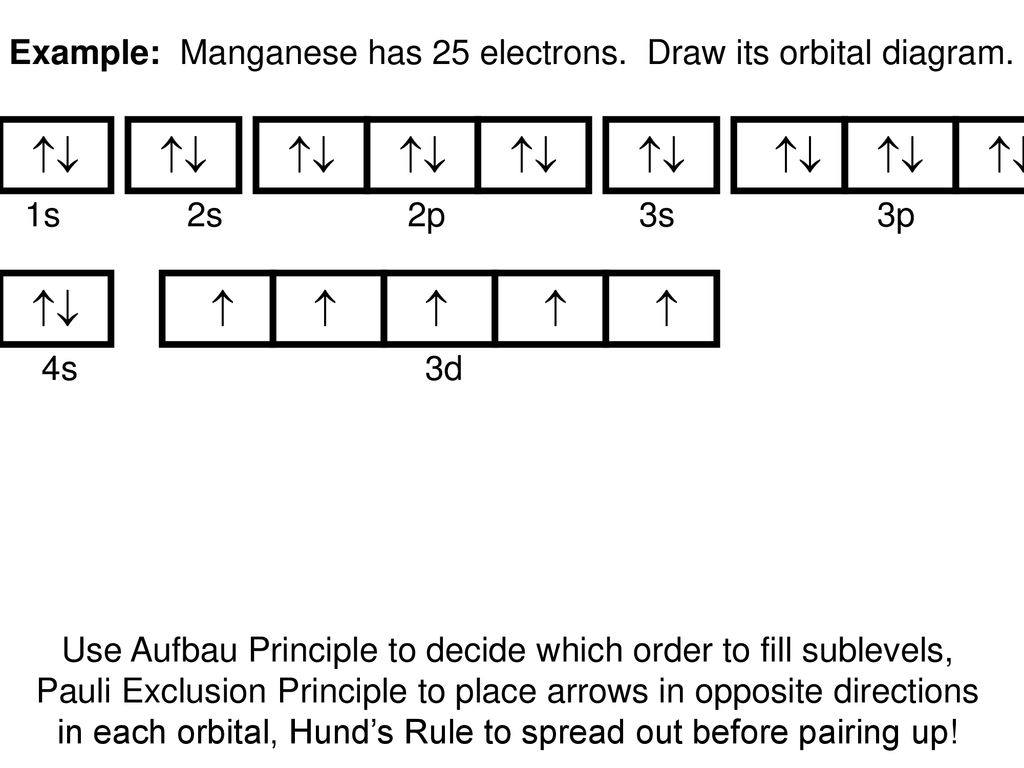
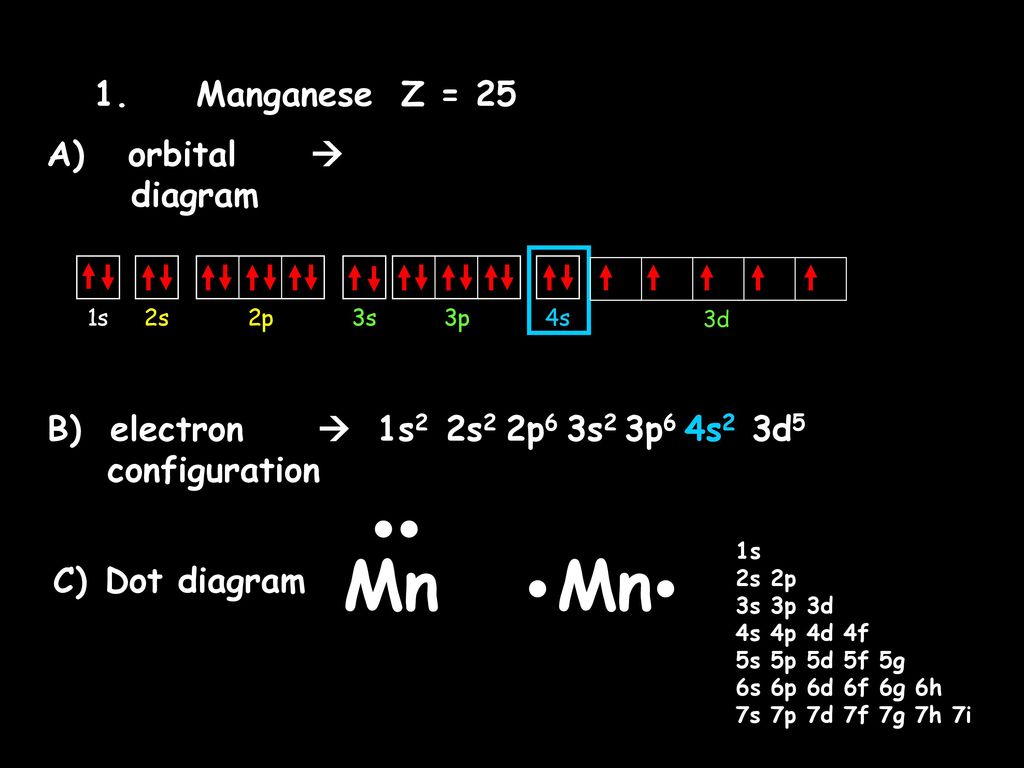
Jul 29, 2016 · Refer to the explanation. The electron configuration of manganese, atomic number 25, is "1s"^2"2"^2"2p"^6"3s"^2"3p"^6"3d"^5"4s"^2". The diagram below represents the electron configuration as an orbital diagram.
Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium ...
Show transcribed image text. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (2 ratings) Transcribed image text: stion 15 Part A Which is the correct orbital diagram for manganese? 3d Submit Request Answer MW.
Write the orbital diagram for sulfur and determine its number of unpaired electrons. Electron configuration: 1s2 2s2 2p6 3s2 3p4. Orbital diagram: 1s= 1 up 1 down. 2s= 1 up 1 down. 2p= 1 up 1 down 1 up 1 down 1 up 1 down. 3s= 1 up 1 down. 3p= 1 up 1 down 1 up 1 up. Two unpaired electrons. Write the electron configuration for Ge.
Doesn't the 5s come before the 4d? I chose [Kr]5s^2 on a quiz but it was wrong. Therefore, the ground state electron configuration for Zr 2+ is : [Kr]4d 2 5s 2. In^+1 [Kr] 5s2 4d10. 1s2, 2s2, 2p6. 1) nuclear charge and relative energy of 3d and 4s orbitals, 2) relative e-e repulsions in 3d and 4s orbitals, 3) exchange energy. Booster Classes. Need an editable periodic table to edit? Enter the ...
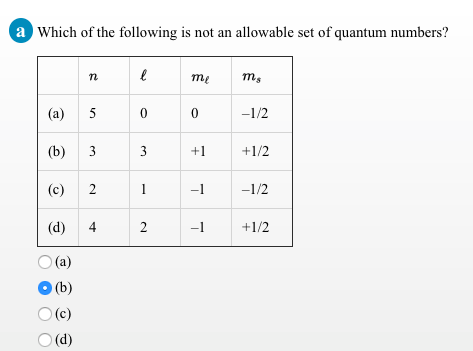
4. Spin Quantum Number (ms): m s = +½ or -½. Specifies the orientation of the spin axis of an electron. An electron can spin in only one of two directions (sometimes called up and down). The Pauli exclusion principle (Wolfgang Pauli, Nobel Prize 1945) states thatno two electrons in the same atom can have identical values for all four of their quantum numbers.
1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^5 is the orbital notation for manganese. Orbital notation requires arrows denoting the spin of each electron. For the purposes of the answer, I'll simply provide ...
When the manganese atom is oxidized, it becomes more electronegative. In the +7 oxidation state, this atom is electronegative enough to react with water to form a covalent oxide, MnO 4-.. It is useful to have a way of distinguishing between the charge on a transition-metal ion and the oxidation state of the transition metal.
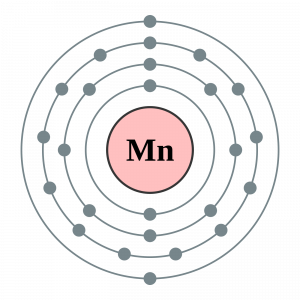
see below it is Mn (manganese) N= 3 third energetic level l= 3 shape of orbitals of type d : (quatrefoil) 5 = the 5 orbitals d are all full means with only one electron in every orbitals (M,magnetic orbitatals = -2,-1, 0 +1 +2 what is the last, you don't know because are isoenergetic) s =intrinsic magnetic moment = +- h/(2 xx pi)(what is the last, you don't know, for convention the negative one)
required and write a partial orbital diagram. PROBLEM: Use partial orbital diagrams to describe how mixing of the atomic orbitals of the central atom(s) leads to hybrid orbitals in each of the following: (a) Methanol, CH. 3. OH (b) Sulfur tetrafluoride, SF. 4 (a) CH. 3. OH. The electron- group arrangement is tetrahedral around both the C and ...
The element manganese (symbol = Mn) has five valence electrons. FALSE. Bromine has 17 valence electrons. ... Which one of the following is the correct orbital diagram for nitrogen? Ar. ... Choose the answer that best completes the following statement: When an aluminum atom reacts so as ...
In picture 1 we show the molecular orbital structure of F2. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. The dashed lines show the remaining p orbitals which do not take part in the bonding. σ z y x σ* x y z Construct the molecular orbital diagram for ...
Sulfur Orbital Diagram - High Concentrations Manganese And Sulfur In Deposits Murray. arrangements of electrons in the orbitals of an atom is the orbital diagram manganese the additional electron is added to plete the half filled 4s sublevel and the configuration is [ar]4s 2 3d 5. Molecules 21 g005.
Ground State Electron Configuration For Nitrogen. When we talk about the electronic configuration, then the ground state Nitrogen Electron Configuration is written as 1s 2 2s 2 2p 3.Below you can get the full image representation which will help you to understand the topic more easily.
41 orbital diagram for titanium. The electron configuration for titanium is 1s22s22p63s23p63d24s2, according to the Jefferson Lab website. The element's 22 electrons are arranged in four energy levels surrounding the nucleus of the atom. Electrons orbit the nucleus in energy levels, which are also called shells.
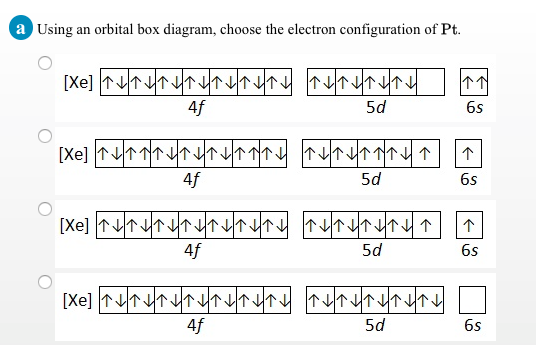
Nickel is a chemical element with atomic number 28 which means there are 28 protons and 28 electrons in the atomic structure.The chemical symbol for Nickel is Ni. Electron Configuration and Oxidation States of Nickel. Electron configuration of Nickel is [Ar] 3d8 4s2. Possible oxidation states are +2,3. Electron Configuration
Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2.
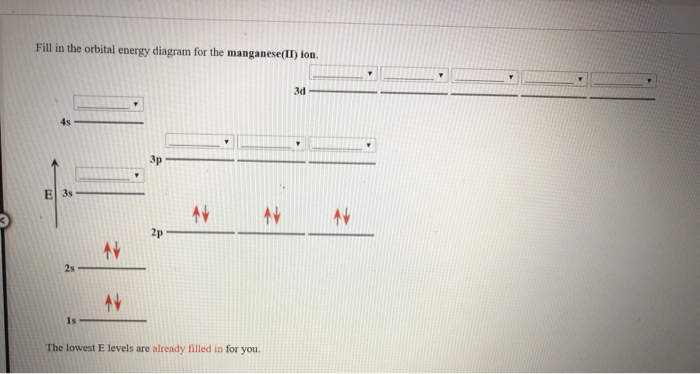
Give the complete electronic configuration for Mn. Choose the statement that is TRUE. *Core electrons effectively shield outer electrons from nuclear charge. *Core electrons are the easiest of all electrons to remove. *Outer electrons efficiently shield one another from nuclear charge. *Valence electrons are most difficult of all electrons to ...
Azimuthal quantum number, l = 0 (since for s orbital l = 0) Magnetic quantum number, m = 0 (since for l = 0, there is only one m value i.e. 0) Spin quantum number, s = +½ or -½ (there is only one electron and it can have clockwise or anticlockwise spins) Conclusion: The correct set of quantum numbers will be: 5, 0, 0, +½ or -½. Therefore ...






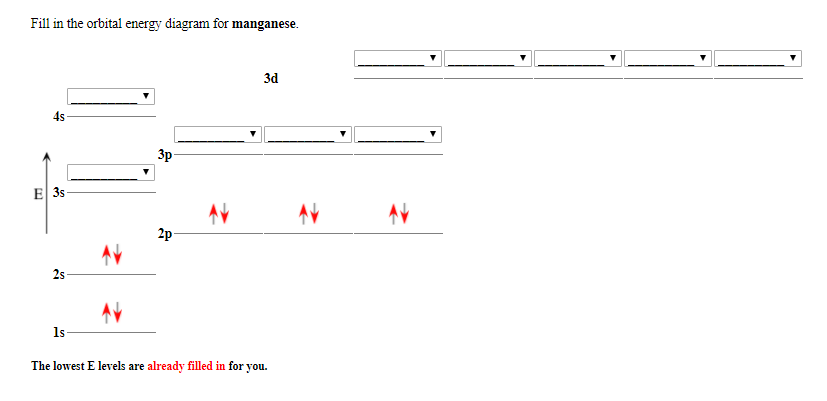









0 Response to "37 choose the correct orbital diagram for manganese."
Post a Comment