37 c2 molecular orbital diagram
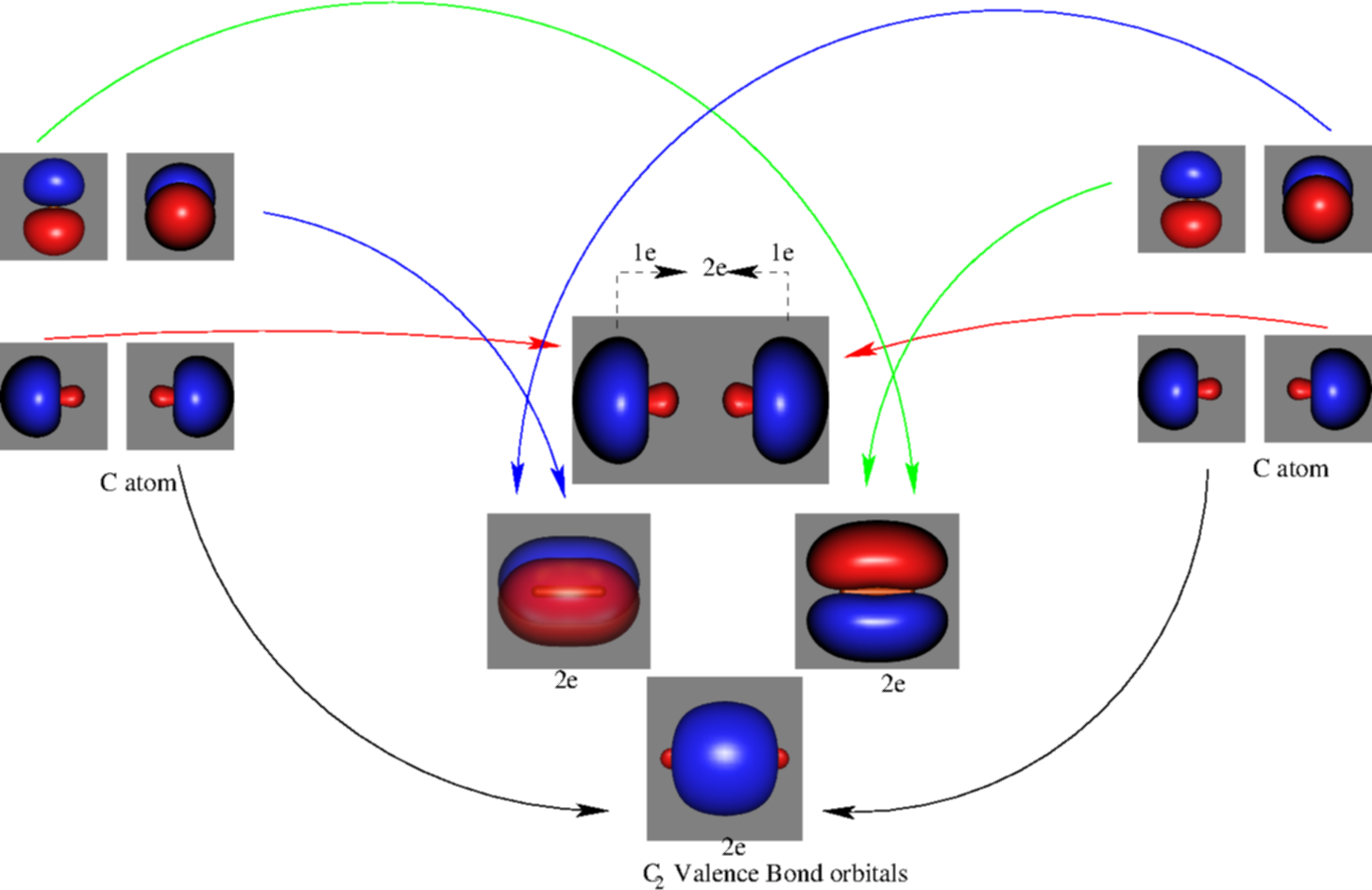
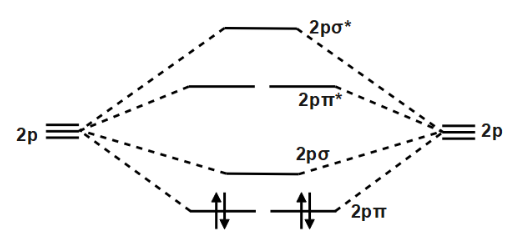
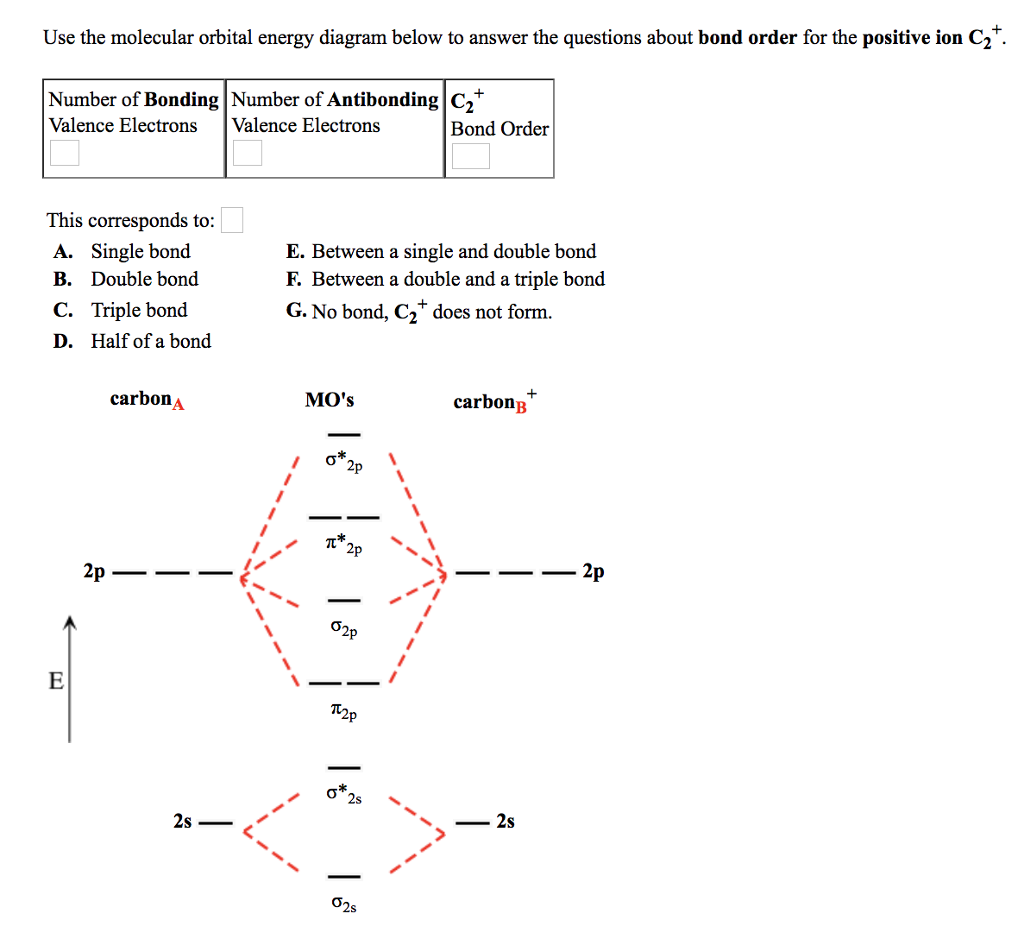
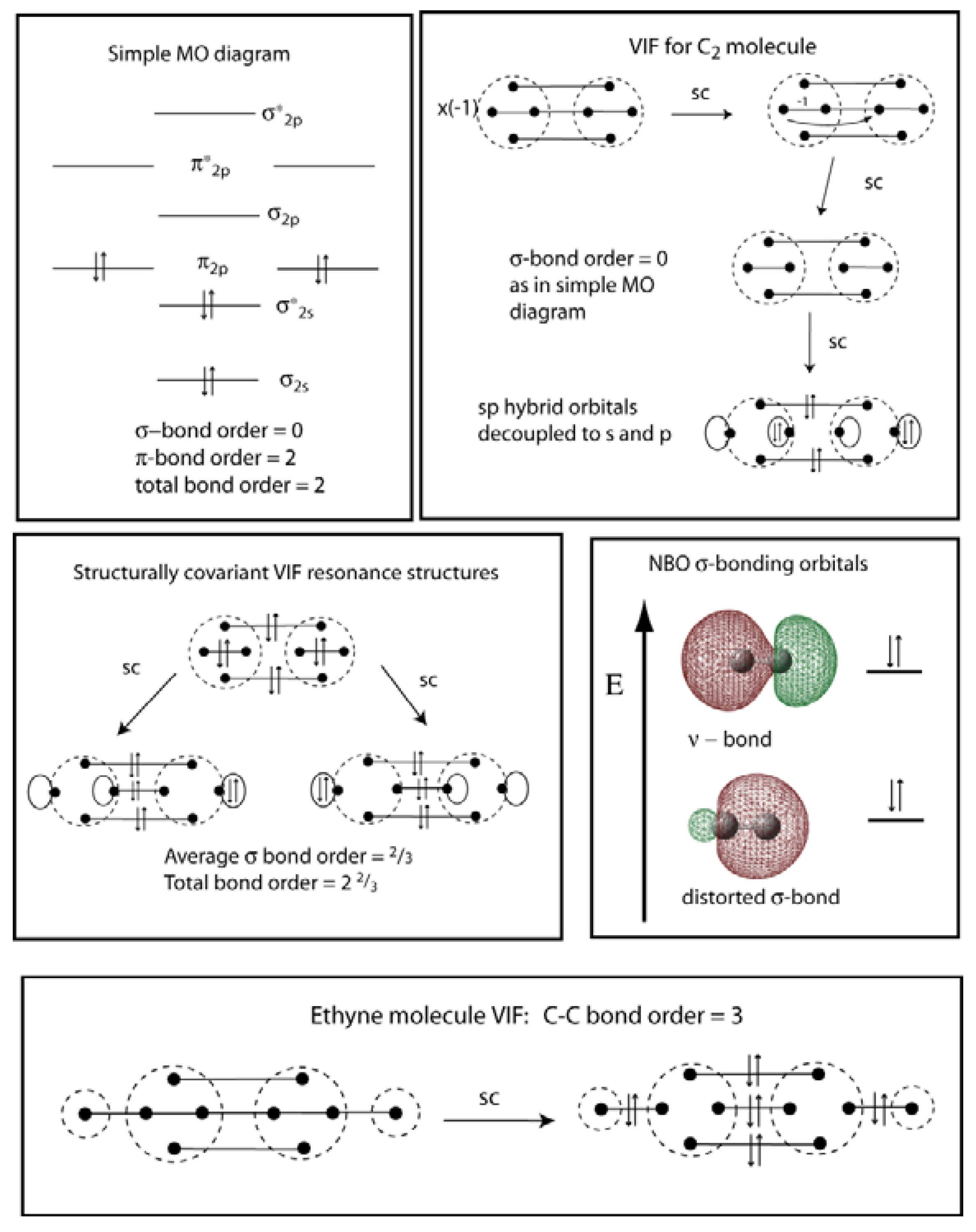
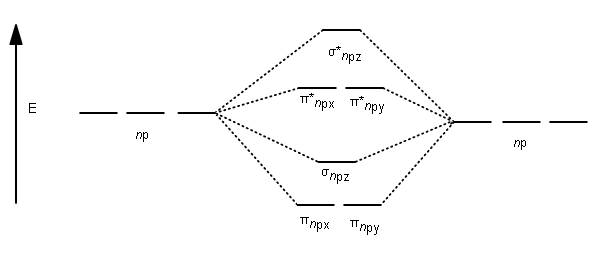
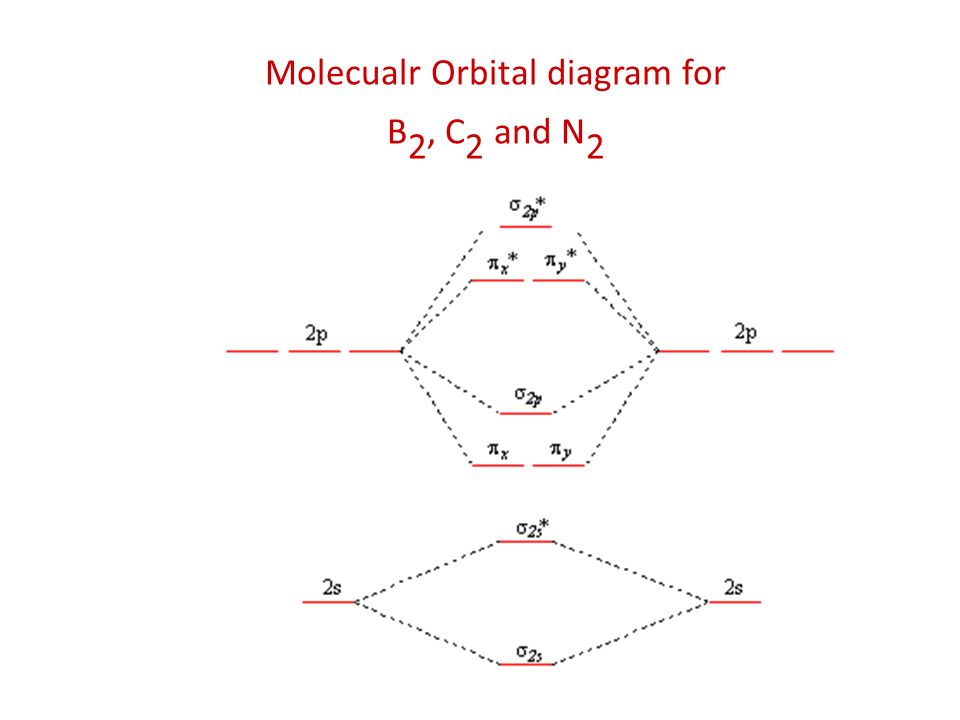
Frontier molecular orbital theory has also been used to explain the regioselectivity patterns observed in Diels–Alder reactions of substituted systems. Calculation of the energy and orbital coefficients of the components' frontier orbitals [17] provides a picture that is in good accord with the more straightforward analysis of the substituents' resonance effects, as illustrated below. When two carbons atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals.C2(2-) has a bond order of 3, so i...
Orbital-orbital Interactions and Symmetry Adapted Linear Combinations; ... Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals. Explore bonding orbitals in other small molecules. Hydrogen ...
C2 molecular orbital diagram
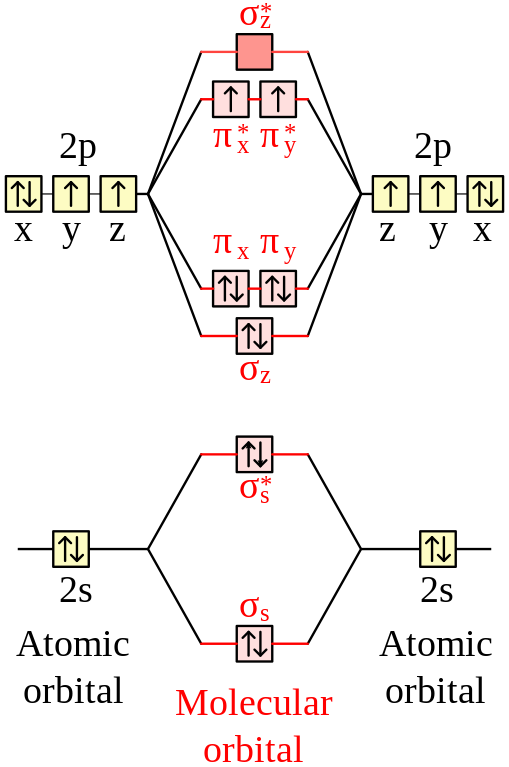
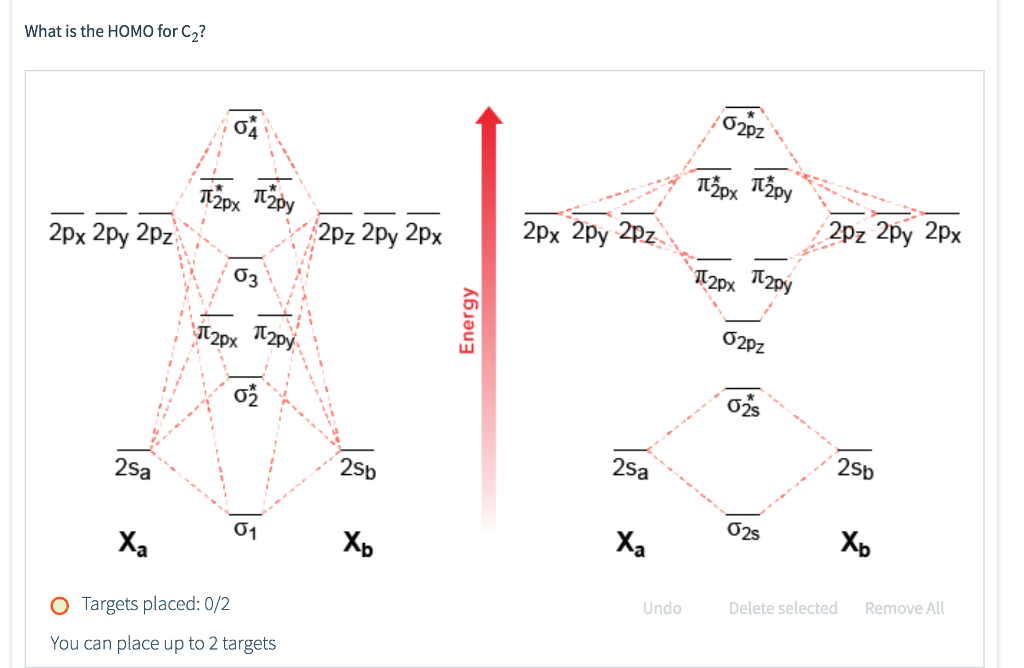
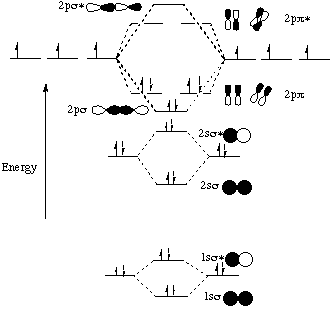
Dec 14, 2014 · The answer is C2- because of bond orders. When we draw the C2 MO, we have everything up till the PiPy Orbitlal filled, and the next orbital tht would be filled would be the sigma2Pz orbital. As for bond orders it is 1/2* [ (#e- in bonding orbitals)- (#e- in antibonding orbitals)] Doing this, normally just C2 is 1/2* [ (8)-4]=2. 31.10.2021 · In pursuit of energetic materials which have equal energies and thermal stabilities, but lower sensitivities compared with hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX), a new fused N-heterocyclic framework, ditriazolo-1,2,4,5-tetrazine, 7 was designed and synthesized. Compound 7 exhibits a favorable … HOMO: the highest occupied molecular orbital of a molecule, ion or atom. homolytic reaction: a reaction in which a covalent bond is broken with equal sharing of the electrons from the bond. hybridization: the process whereby atomic orbitals of different type but similar energies are combined to form a set of equivalent hybid orbitals.
C2 molecular orbital diagram. ʻOumuamua is small and not very luminous. It was not seen in STEREO HI-1A observations near its perihelion on 9 September 2017, limiting its brightness to approximately 13.5 mag. By the end of October, ʻOumuamua had already faded to about apparent magnitude 23, and in mid-December 2017, it was too faint and fast moving to be studied by even the largest ground … A) The total number of molecular orbitals formed doesn't always equal the number of atomic orbitals in the set. B) A bond order of 0 represents a stable chemical bond. C) When two atomic orbitals come together to form two molecular orbitals, one molecular orbital will be lower in energy than the two separate atomic orbitals and one molecular orbital will be higher in … Construct the molecular orbital diagram for N2. The molecular orbital diagram for N 2. The molecular orbitals from lowest energy to highest energy are one sigma 2 s orbital, one sigma 2 s star orbital, two pi 2 p orbitals, one sigma 2 p orbital, two pi … 24.11.2021 · We investigated the chemical pressure effects on structural and electronic properties of SnTe-based material using partial substitution of Sn by Ag0.5Bi0.5, which results in lattice shrinkage. For ...
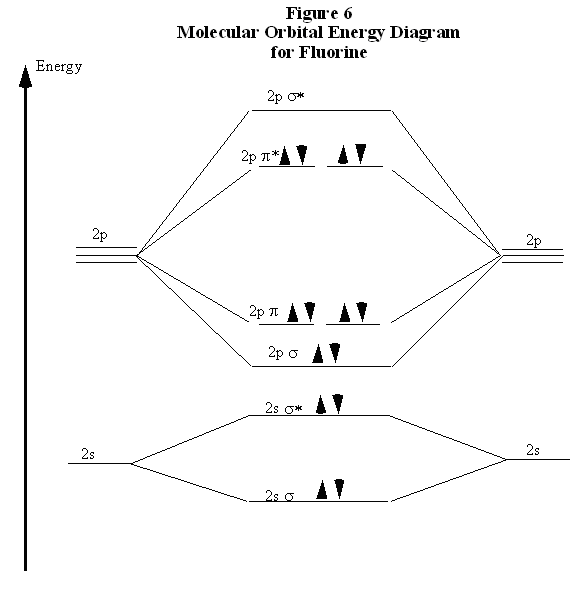
Feb 26, 2018 · The molecular orbital diagram for C 2 molecule is :. The electronic configuration of C 2 is K K (σ2s) 2 (σ * 2s) 2 n(2px) 2 n(2py) 2. The C 2 molecule is diamagnetic because all electrons are paired there are no unpaired electrons. Stereoisomers. As defined in an earlier introductory section, isomers are different compounds that have the same molecular formula. When the group of atoms that make up the molecules of different isomers are bonded together in fundamentally different ways, we refer to such compounds as constitutional isomers.For example, in the case of the C 4 H 8 hydrocarbons, … Oct 17, 2018 · Molecular orbital diagram for c2. This video shows the mo diagrams of the c2 n2 o2 and f2 molecules. Molecular orbitals are formed combining similar atomic orbitals. Just because some chemical species shows integral value of bond order doesnt mean that it should exist. Molecular orbital diagram for the molecule oxygen o2. Dec 02, 2016 · The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram. C2:(1sσ)2(1s* σ)2(2sσ)2(2s* σ)2(2pπ)4. The electron configuration of the C− 2 ion will be.
C2 molecular orbital diagram. A mo is defined as the combination of atomic orbitals. As for bond orders it is 12e in bonding orbitals e in antibonding orbitals. The only orbitals that are important in our discussion of molecular orbitals are those formed when valence shell orbitals are combined. QUESTION 1 The molar mass of a gas is determined as in the experiment described in Experiment 8. The mass is determined to be 2.01 g. The volume of the water, which is equal to the volume of the gas, is measured to be 243 mL, the boiling water temperature was 99.8°C and the barometric pressure was 755 mm Hg. HOMO: the highest occupied molecular orbital of a molecule, ion or atom. homolytic reaction: a reaction in which a covalent bond is broken with equal sharing of the electrons from the bond. hybridization: the process whereby atomic orbitals of different type but similar energies are combined to form a set of equivalent hybid orbitals. 31.10.2021 · In pursuit of energetic materials which have equal energies and thermal stabilities, but lower sensitivities compared with hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX), a new fused N-heterocyclic framework, ditriazolo-1,2,4,5-tetrazine, 7 was designed and synthesized. Compound 7 exhibits a favorable …
Dec 14, 2014 · The answer is C2- because of bond orders. When we draw the C2 MO, we have everything up till the PiPy Orbitlal filled, and the next orbital tht would be filled would be the sigma2Pz orbital. As for bond orders it is 1/2* [ (#e- in bonding orbitals)- (#e- in antibonding orbitals)] Doing this, normally just C2 is 1/2* [ (8)-4]=2.

















0 Response to "37 c2 molecular orbital diagram"
Post a Comment