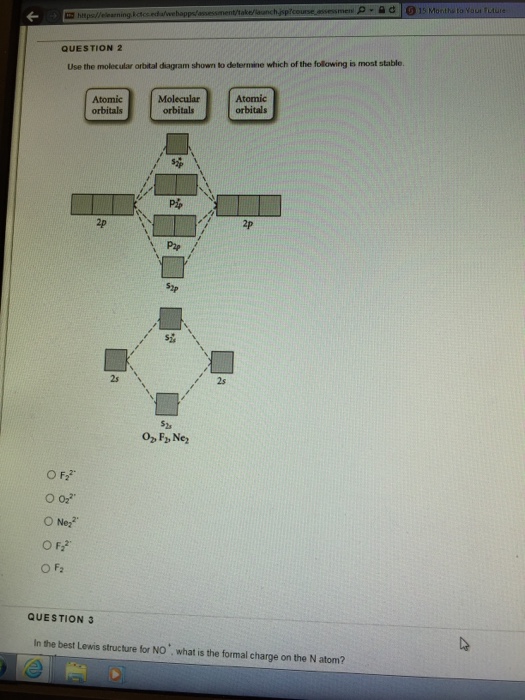
36 draw the molecular orbital diagram shown to determine which of the following is most stable.
Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution We draw a molecular orbital energy diagram similar to that shown in .
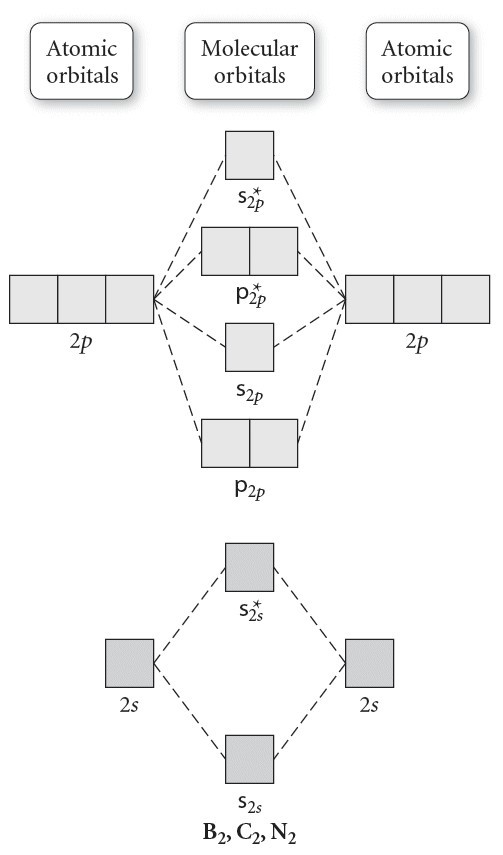
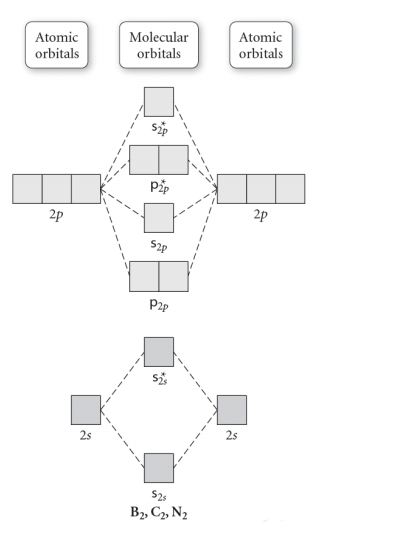
Molecular Orbital Energy Diagrams. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right.
Draw the molecular orbital diagram shown to determine which of the following is most stable. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. C22- should have the highest bond order (3, it has 6 more e ….

Draw the molecular orbital diagram shown to determine which of the following is most stable.
We're being asked which species is the most stable. For this, we need to determine the bond order for each species. The bond order tells us the stability of a bond: a higher bond order means the bond is more stable. Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram.
B f22 c ne22 d o22 e f22 2 use molecular orbital diagrams to determine which of the following are paramagnetic. Use the molecular orbital diagram shown to determine which of the following is most stable. Use the molecular orbital diagram shown to determine which of the following is most stable. D c 2 2. Start studying exam 3.
Draw the molecular orbital diagram shown to determine which of the following is most stable. O22+ Identify the number of electron groups around a molecule with a : tetrahedral shape
Draw the molecular orbital diagram shown to determine which of the following is most stable..
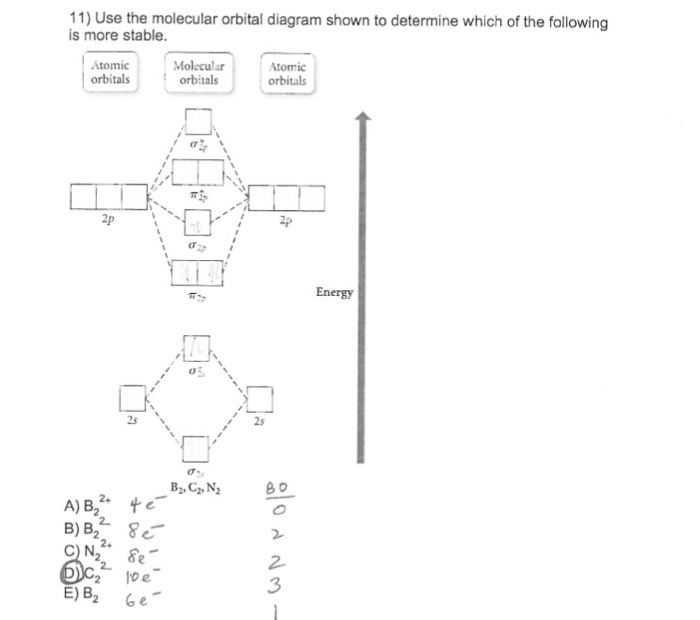
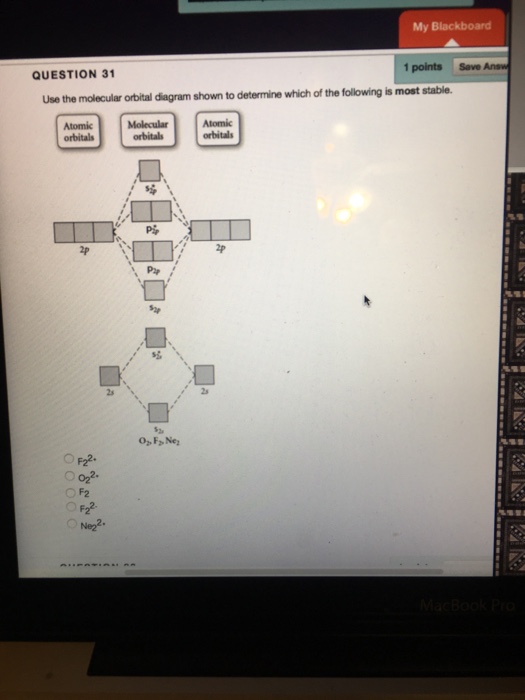
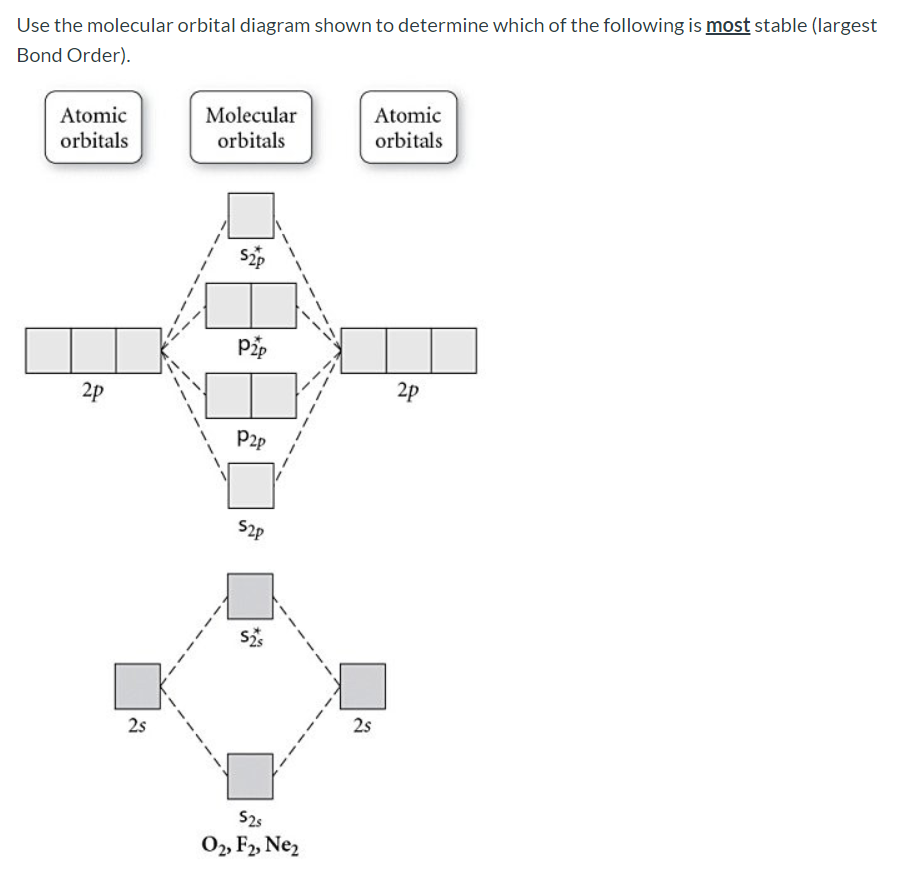
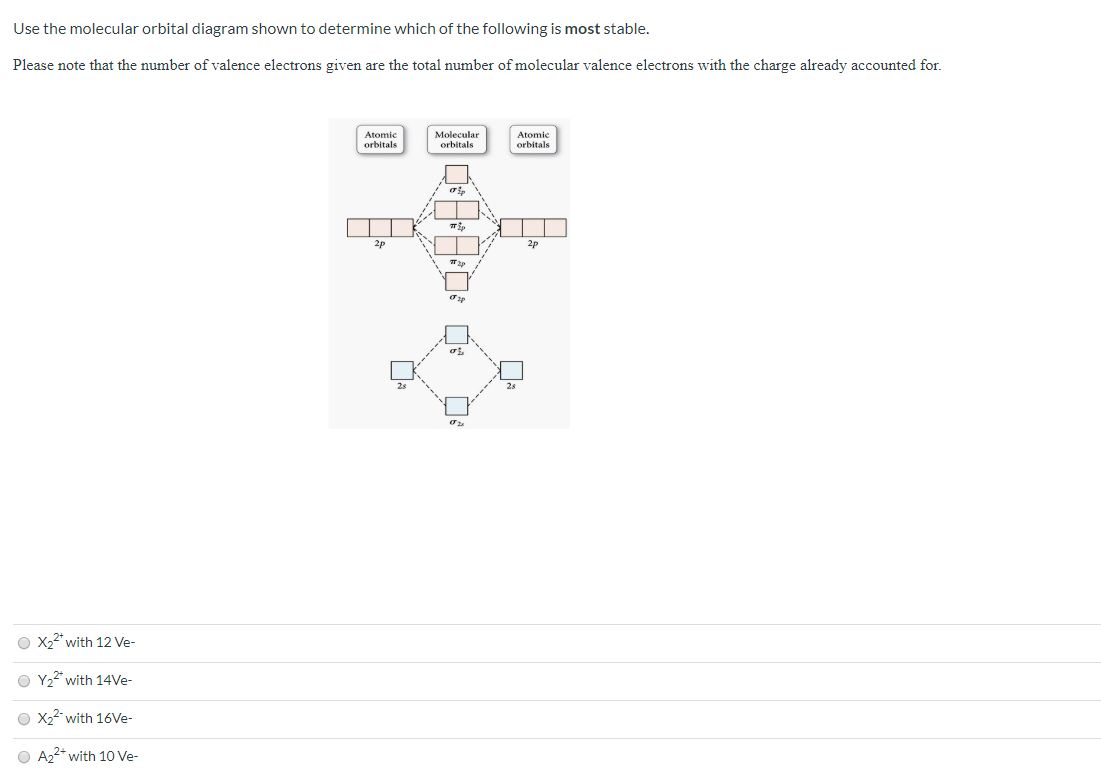
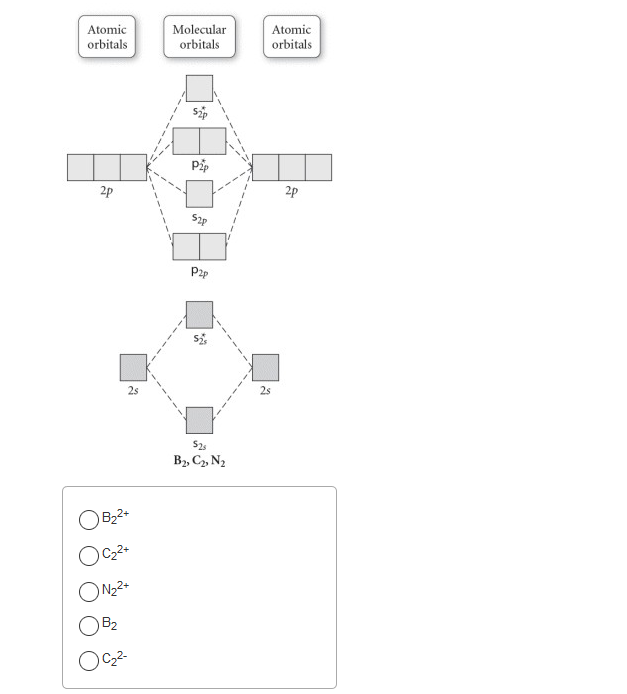
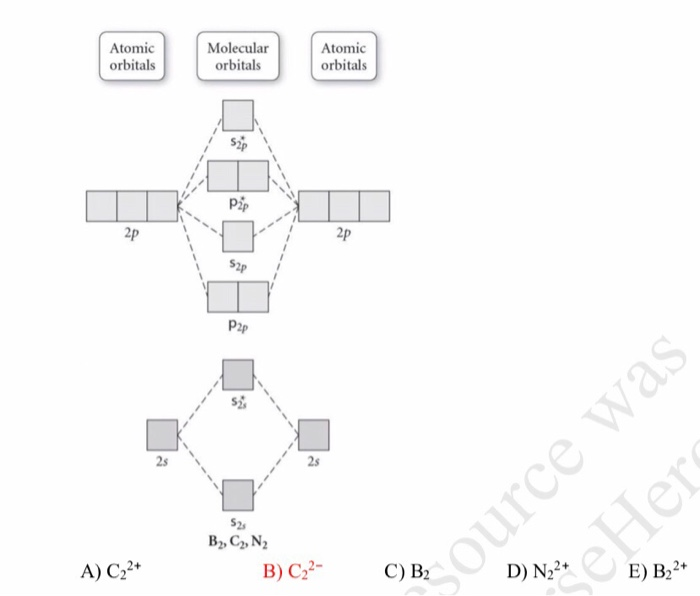
31) Use the molecular orbital diagram shown to determine which of the following is most stable. A) C2^2⁺ B) N2^2⁺ C) B2; D) C2^2⁻ E) B2^2⁺ 32) How many moles of oxygen are formed when 58.6 g of KNO3 decomposes according to the following reaction? The molar mass of KNO3 is 101.11 g/mol. 4 KNO3(s) → 2 K2O(s) + 2 N2(g) + 5 O2(g)
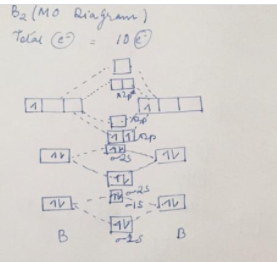
Molecular orbital energy diagram of b2. Molecular orbital energy diagram of b2
Dec 11, 2019 · Fill in the Molecular Orbital Energy Diagram for the diatomic molecule Ne 2. Draw the atomic and hybrid orbitals on on side of the page. A) B2^2+ B) B2^2-C) N2^2+ D) C2^2-E) B2; 4) Draw the molecular orbital diagram shown to determine which of the following is most stable. Hence the bond order is 1/2(bonding- anti bounding ...
more stable than on the individual atoms, this is referred to as a bonding molecular orbital. A second molecular orbital is also created, which we simplistically show as a subtraction of the two atomic 1s orbitals [σ* = (1sa - 1sb)]. This orbital is called sigma-star (σ*) and is less stable than the two separated atoms. Because it is less ...
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
Use the molecular orbital diagram shown to determine which of the following is most stable. The three 3 component mixture shown below was spotted to a tlc plate and developed using the solvent system listed. 1 draw the molecular orbital diagrams to determine which of the following is most stable. Lindau 3 years ago 0.
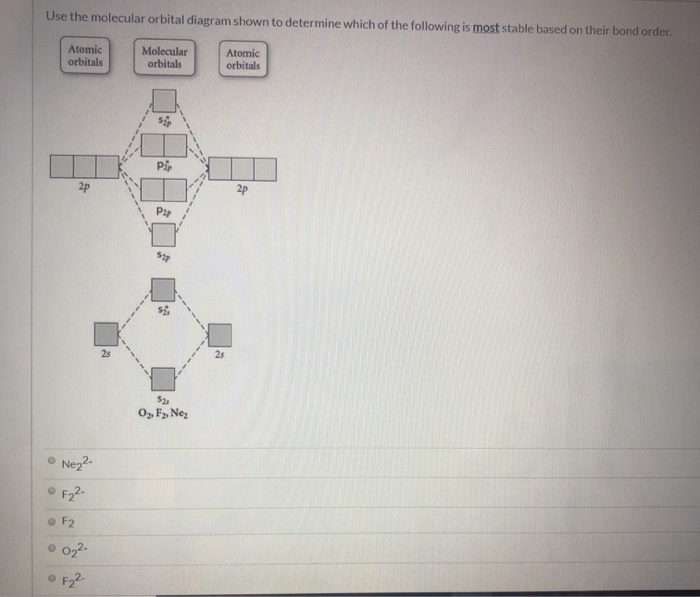
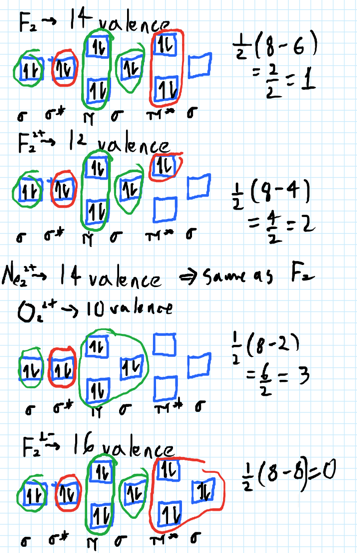
Problem: Use the molecular orbital diagram shown to determine which of the following is most stable.a. F22+b. Ne22+c. F22-d. O22+e. F2 FREE Expert Solution Recall that the bond order t ells us the strength and length of a bond: a higher bond order means the bond is stronger and shorter.
3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic. A) B2^2+ B) B2^2-C) N2^2+ D) C2^2-E) B2; 4) Draw the molecular orbital diagram shown to determine which of the following is most stable. A) C2^2+ B) N2^2+ C) B2; D) C2^2-E) B2^2+ 5) Which statement regarding stable heteronuclear diatomic ...
Molecular orbital diagram for b2. By drawing molecular orbital diagram s for b2 c2 n2 o2 and f2 predict which of these homonuclear diatomic molecules are magnetic. The molecular orbital diagram for an o 2 molecule would there for e ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbital s.. OM diagram for the N2 molecule.
Which statement is true? a. The total number of molecular orbitals formed doesn't always equal the number of atomic orbitals in the set. b. When two atomic orbitals come together to form two molecular orbitals, one molecular orbital will be lower in energy than the two separate atomic orbitals and one molecular orbital will be higher in energy than the separate atomic orbitals.
Use the molecular orbital diagram shown to determine which of the following is LEAST stable. B2⁺ The highest energy occupied molecular orbital in the F—F bond of the F2 molecule is _____. Draw the molecular orbital diagram shown to determine which of the following is MOST stable. A) B2^2+. B) C2^2+. C) N2^2+. D) C2^2-
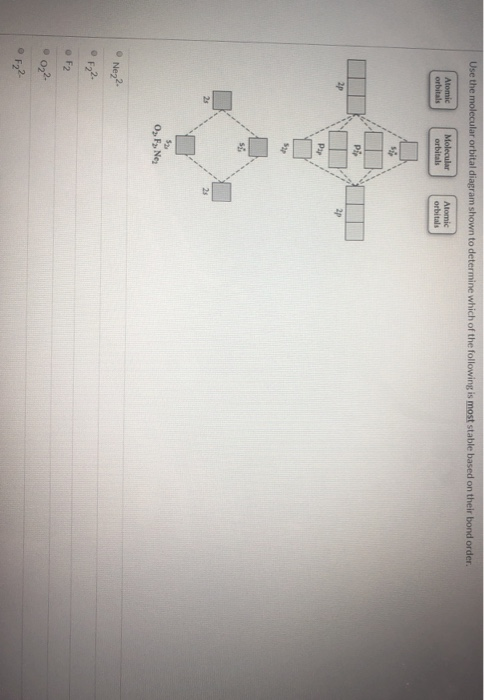
Draw the molecular orbital diagram shown to determine which of the following is most stable. N22 c22 c22 b22 b2. 1 draw the molecular orbital diagrams to determine which of the following is most stable. F22 identify the number of electron groups around a central atom with sp2 hybridization. Determine the magnetism of simple molecules.
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
Molecular orbital diagram of b2. Draw the molecular orbital energy level diagram for each of the following species Be2+, Be2, and Be Indicate theirnumbers of unpaired electron and mention. Each sp 1 hybrid orbital has s-character and The molecular orbital structure of ethylene: In ethene molecule, each carbon atom undergoes sp 2 hybridisation. 3.
E 2 bonding pairs and 2 lone pairs. 1 draw the molecular orbital diagrams to determine which of the following is most stable. By constructing a molecular orbital picture for each of the following molecules determine whether it is paramagnetic or diamagnetic.
Draw the Molecular orbital Diagram Shown to Determine which Of the Following is Most Stable. use the molecular orbital diagram shown to determine which use the molecular orbital diagram shown to determine which of the following is most stable 38 a b2 b c22 use the molecular orbital question draw the molecular orbital diagram shown to answer to draw the molecular orbital diagram shown to ...
Using the Frost Circle mnemonic, draw the molecular orbital diagram for a cyclopentadienyl carbanion (energy levels only). Indicate which MOs are bonding, non-bonding and anti-bonding. Use up and down arrows to represent the electrons present in each orbital. State whether a cyclopentadienyl carbanion is aromatic, anti-aromatic or neither.
1. Draw the molecular orbital diagram of C2 2+ and C2 2- (atomic number = 6) C2 2+ C2 2- a) What is paramagnetic? (1 pt.) b) Which one has the highest bond ...4 answers · Top answer: So here we're looking at the molecular orbital theory to describe bonding. So the first example, ... 26.11.2021 · 2. 1.1. Molecular design of B/L biacidic ILs. In this article, cellobiose was used as the model ...
Give the electron configurations for the species C2 and C When we draw the C2 MO, we have everything up till the PiPy Orbitlal filled, and the next orbital tht would be filled would be the sigma2Pz. Answer to Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. N22+ B22+ B B2 CeV.
The most stable element based on the molecular orbital diagram is . Explanation: Molecular Orbital Theory (MOT) was proposed by Hund and Mulliken. The theory describes the bonding in molecules, elements, or atoms. The theory uses Molecular Orbital (MO) diagram to explain the bonding between atoms. For example, to determine the stability of an ...

Use the molecular orbital diagram shown to determine which of the following is most stable.a) n22+ b) b2c) b22+d) c22-e) c22+

Use the molecular orbital diagram shown to determine which of the following are paramagnetic.a. ne22+ b. o22+ c. f22+ d. o22- e. none of the above are paramagnetic.














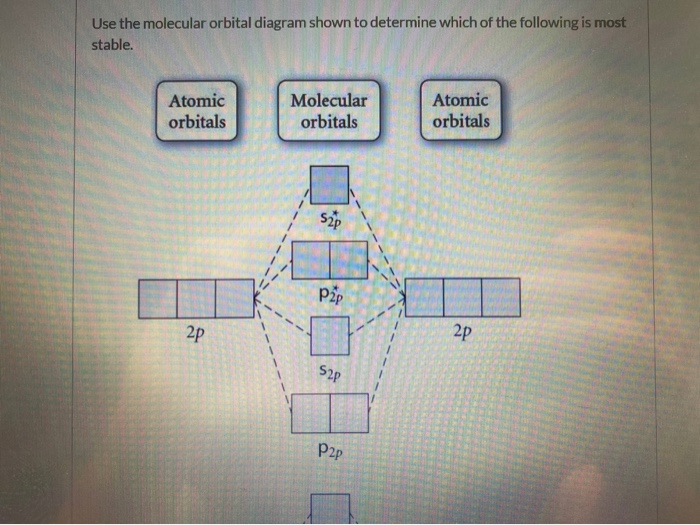
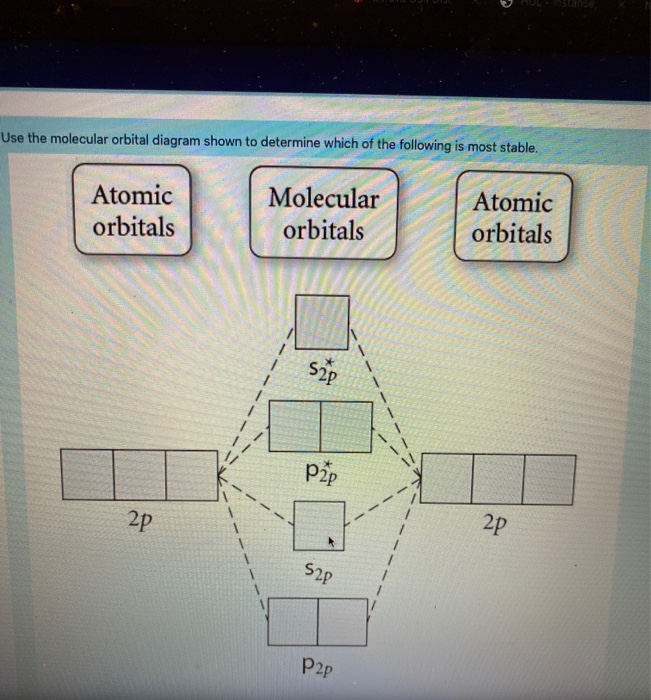

0 Response to "36 draw the molecular orbital diagram shown to determine which of the following is most stable."
Post a Comment