42 magnesium lewis dot diagram
A step-by-step explanation of how to draw the MgO Lewis Dot Structure.For MgO we have an ionic compound and we need to take that into account when we draw th... Magnesium Dot Diagram. what is the lewis dot diagram for magnesium quora draw a magnesium symbol and dot two dots around the symbol hopes this helps high school chemistry lewis electron dot diagrams high school chemistry lewis electron dot electron dot formula for magnesium title=high school chemistry lewis electron dot diagrams&oldid
Magnesium nitride (Mg3N2 or N2Mg3) is IONIC because it is a combination of a metal and non-metal.Each magnesium, of which there are three, LOSES two electron...
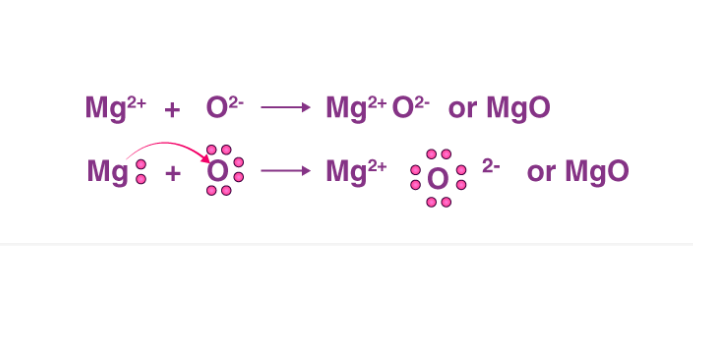
Magnesium lewis dot diagram
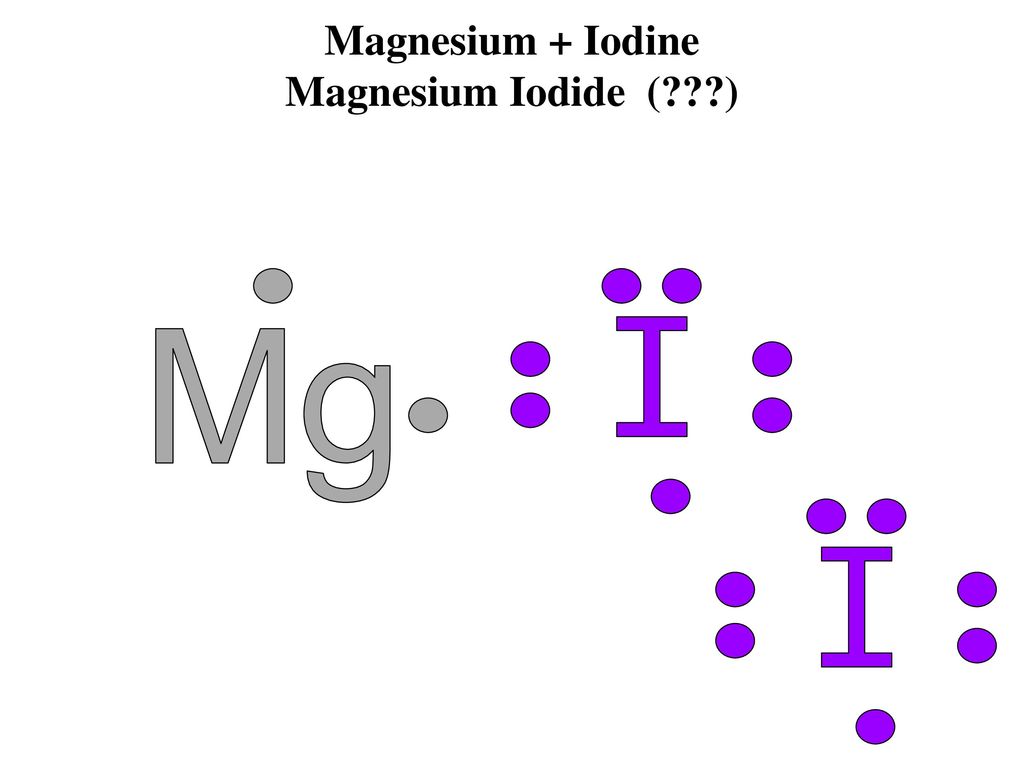
Magnesium Iodide (MgI2 or I2Mg) is an ionic compound.One magnesium atom loses two electrons, so it becomes +2 chargeTwo iodine atoms gain those two electrons... Magnesium phosphide (Mg3P2 or P2Mg3) is IONIC because it is a combination of a metal and non-metal.Each magnesium, of which there are three, LOSES two electr... This is the correct dot diagram for sodium, group 1. Q. This is the correct dot diagram for nitrogen, group 15. Q. This is a correct dot diagram for neon, group 18. Q. This could be the dot diagram of. Mg, group 2. Cl, group 17.
Magnesium lewis dot diagram. It is MgBr2. Magnesium has an electronic configuration of 2.8. 2. It has to lose 2 electrons to attain a stable electronic configuration and octet valance shell structure resembling that of noble gases. What is the correct Lewis dot diagram for MgBr2? Magnesium Fluoride Lewis Dot Diagram. Magnesium fluoride is prepared from magnesium oxide with sources of hydrogen fluoride such as ammonium bifluoride. This video shows you how to draw the lewis structure for ionic compounds such as NaCl - sodium chloride, MgF2 - magnesium fluoride, or Al2O3 - aluminum oxide. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. Exercises. 1. Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol. 2. Oct 26, 2019 · Lewis Dot Structure for Magnesium(Mg) Hello,today I am going to draw the lewis Dot structure for magnesium (Mg) in just two steps. Step-1: To draw the lewis Dot structure of magnesium (Mg) , we have to find out the valence electrons of magnesium (Mg) first.We express valence electrons as dots in lewis dot structure.
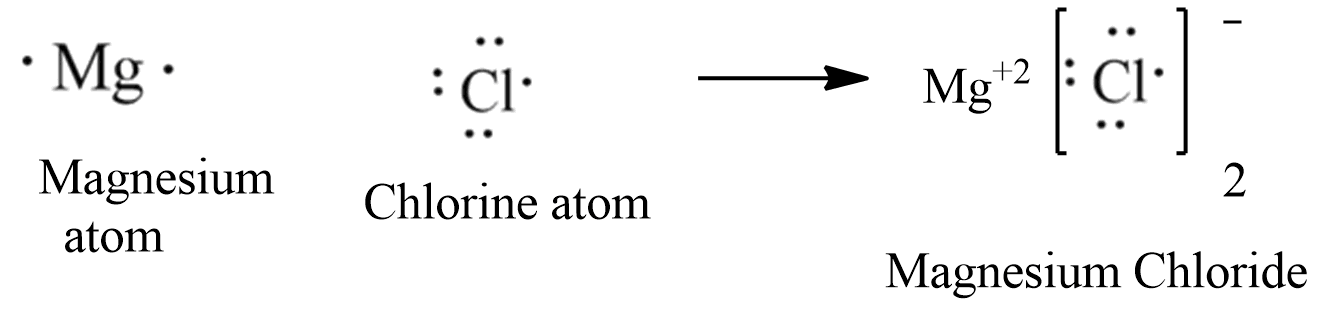
Lewis dot diagram for magnesium chloride. There are two types of diagrams one is the lewis diagram the other is the electron dot diagram. Note that mgcl2 is also called magnesium chloride. A covalent bond in which electrons are shared equally is called a what. Magnesium chloride is a compound which is composed of positive and negative ions. The lewis dot structure is a diagram to show the bonding between the atoms of a molecule and pairs of electrons that may exist. Ionic bonding chemistry for non majors. The electrons are shown as dots on the outside of the atomic symbol. The lewis dot structure for chromium is cr with two dots on top and. Magnesium has two electrons on its outer ... The lewis dot structure for chromium is cr with two dots on top and. Lewis dot diagram for magnesium. Since the lewis electron dot diagrams are based on the number of valence electrons it would hold true that the elements in the same group would have the same electron dot diagram. The electrons are shown as dots on the outside of the atomic symbol. Nov 07, 2021 · The Lewis dot structure for Magnesium is an Mg with 2 dots which stand for its two valence electrons. The Lewis dot structure for Sulfur is an S with 6 dots which stand for its six valence electrons. These two elements when bonded together form an ionic bond as the Magnesium loses its two valence electrons to…
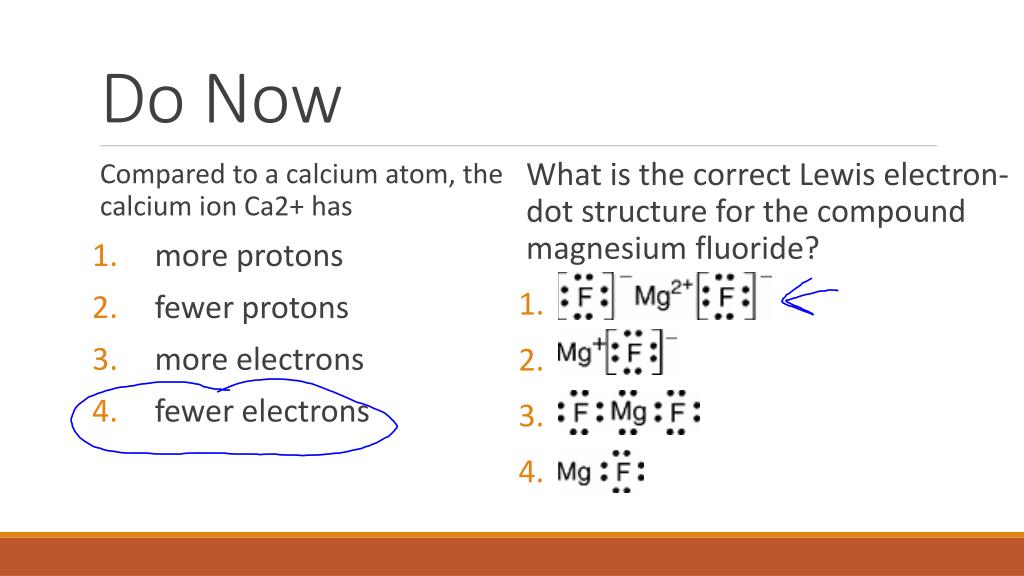
Topic: Lewis Dot Diagrams for Ionic Compounds. Do Now: Identify ionic compounds. CO. 2 MgCl. 2 NH. 4. ClNaOH. NH. 3 CH. 4 CuSO. 4 HF. Magnesium's outer shell is now empty. Fluorine's outer shell is now full. Lewis Diagrams for Ionic Compounds. NaCl's Lewis structure: A step-by-step explanation of how to draw the MgSO4 Lewis Dot Structure.For MgSO4 we have an ionic compound and we need to take that into account when we dra... Jan 26, 2018 · The lewis dot structure is a diagram to show the bonding between the atoms of a molecule and pairs of electrons that may exist. This eight electrons are found in four pairs. I show you where magnesium is on the periodic table and how to determine how many valence electrons magnesium has. Ionic bonding chemistry for non majors. A step-by-step explanation of how to draw the Lewis dot structure for Mg (Magnesium). I show you where Magnesium is on the periodic table and how to determi...
Magnesium And Chlorine Lewis Dot, 35 Lewis Dot Diagram For Chlorine Wiring Diagram Database, Hydroxide Wikipedia, Chemistry Chemical Bonding (17 of 35) Lewis Structures, Science E portfolio: Summary of what I learnt (Chemical
Step 4. Search for the bond forming between the magnesium and oxygen atoms: The ionic bond will be formed between the atoms as magnesium will be donating two of its valence electrons from the 3s shell to fulfill the electron deficiency in the oxygen atom. Step 5. Now draw the Lewis structure of magnesium oxide (MgO): From the diagram, it is ...
Answer (1 of 3): First, hopefully you noticed that magnesium nitride is an ionic compound since Mg is an earth metal (2A column). Ionic compounds are formed by the metal(s) giving electrons to the nonmetal(s). Mg (2A column) has two valence electrons to give up and, in doing so, will develop a +2...
Since the Lewis electron dot diagrams are based on the number of valence electrons, it would hold true that the elements in the same group would have the same electron dot diagram. In other words, if every element in Group 1A has valence electron, then every Lewis electron dot diagram would have one single dot in their Lewis electron dot diagram.
Magnesium is classified as an alkaline earth metal and has 2 hydration shells. The element can be found in abundance in the hydrosphere and in mineral salts such as dolomite and magnesium carbonate.Common dietary sources of magnesium include nuts (cashews, peanuts, almonds), beans, bananas, apples, carrots, broccoli, and leafy greens. Magnesium is an important enzyme cofactor and is essential ...
Draw An Electron Dot Diagram To Show The Formation Of Each Of The Following Compounds I Methane Ii Magnesium Chloride Sarthaks Econnect Largest Online Education Community
Answer (1 of 3): draw a magnesium symbol and dot two dots around the symbol. Hopes this helps:)
Mar 23, 2018 · The lewis dot structure for magnesium is an mg with 2 dots which stand for its two valence electrons. What is the lewis dot structure for aluminum phosphate. Bookmark the lewis dot diagram for magnesium lewis structure lewis dot systems. Lewis dot diagram for magnesium is actually amongst pictures libraries inside our highest pictures gallery.
: F : Mg : F : .. .. Magnesium has two electrons on its outer shell Each of the electrons will be shared with a Florine atom. This results in a compound MgF_2 Each Florine starts with seven electrons around the atom, combining with the Magnesium atom give the Florine eight electrons around each Florine atom. This eight electrons are found in four pairs. shown in the diagram as a pair on top a ...
The Lewis dot structure for Magnesium is an Mg with 2 dots which stand for its two valence electrons. The Lewis dot structure for Sulfur is an S with 6 dots which stand for its six valence electrons. What is the Lewis structure for magnesium oxide? Magnesium oxide is an ionic compound. Its formula unit (MgO) is made from one magnesium atom ...
Which Lewis electron-dot diagram represents an atom in the ground state for a the correct Lewis electron-dot structure for the compound magnesium fluoride?. MgF2 (2 should be subscript) because fluoride is -1 electron and Mg is 2+ d) draw the dot diagram for magnesium fluoride. since this is an.
Since the lewis electron dot diagrams are based on the number of valence electrons it would hold true that the elements in the same group would have the same electron dot diagram. The lewis dot structure for magnesium is an mg with 2 dots which stand for its two valence electrons. Magnesium is in group 2 sometimes called group ii or 2a.

Draw The Step By Step Structures To Obtain The Lewis Dot Structures And Balance The Charges Of The Following A Fe 2s 3 B Mg 3n 2 Study Com
This is the correct dot diagram for sodium, group 1. Q. This is the correct dot diagram for nitrogen, group 15. Q. This is a correct dot diagram for neon, group 18. Q. This could be the dot diagram of. Mg, group 2. Cl, group 17.
Magnesium phosphide (Mg3P2 or P2Mg3) is IONIC because it is a combination of a metal and non-metal.Each magnesium, of which there are three, LOSES two electr...
Magnesium Iodide (MgI2 or I2Mg) is an ionic compound.One magnesium atom loses two electrons, so it becomes +2 chargeTwo iodine atoms gain those two electrons...

Answered Show The Formation Of Mgo By The Transfer Of Electrons In The Two Elements Using Electron Brainly In
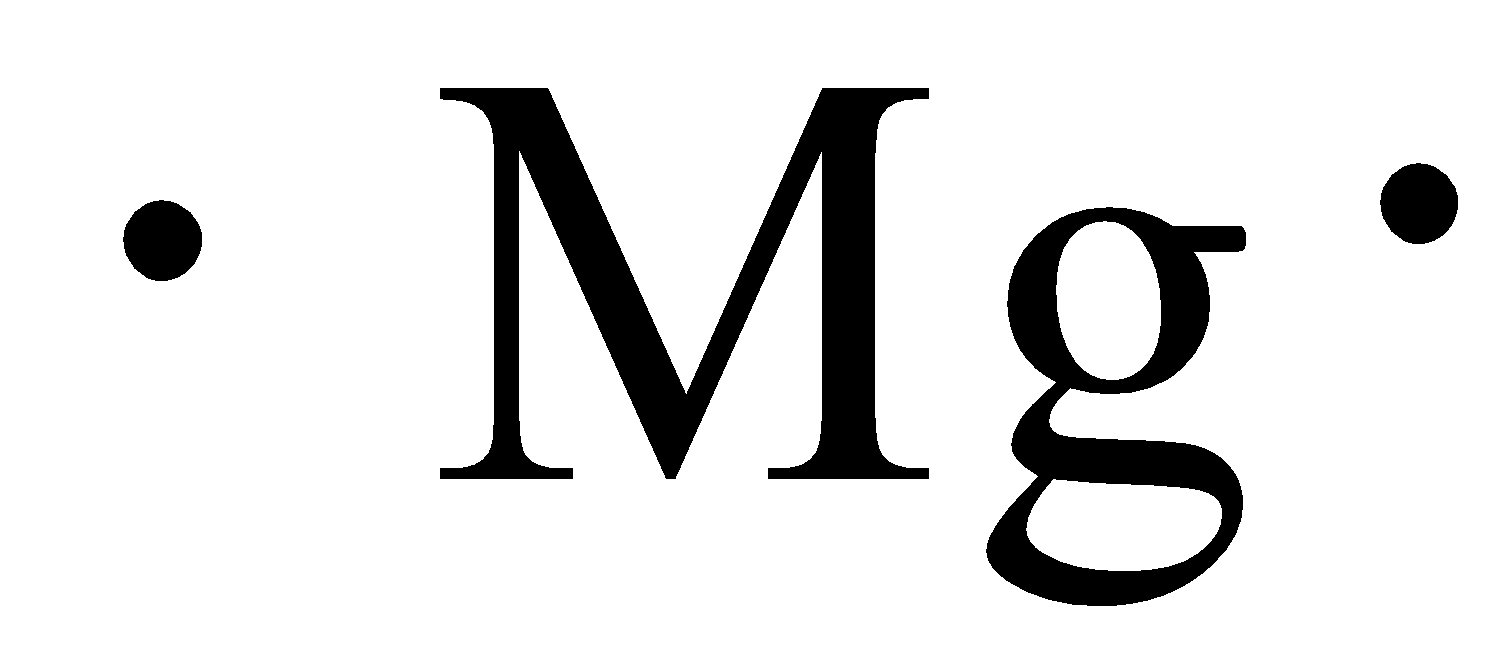
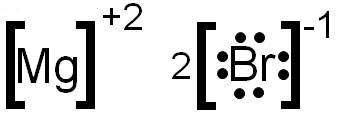






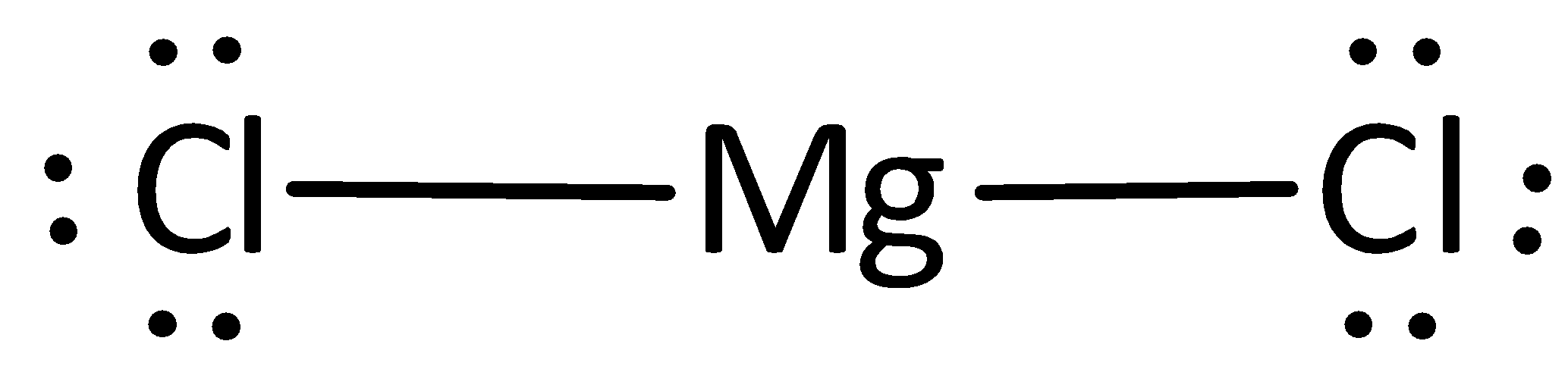

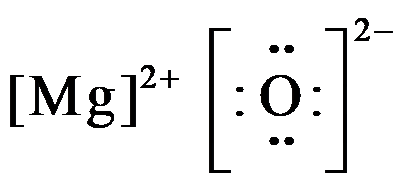









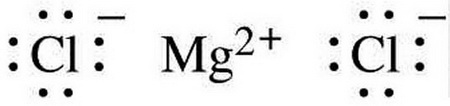
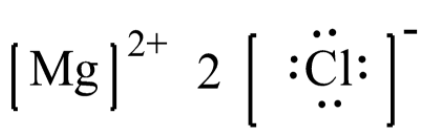
0 Response to "42 magnesium lewis dot diagram"
Post a Comment