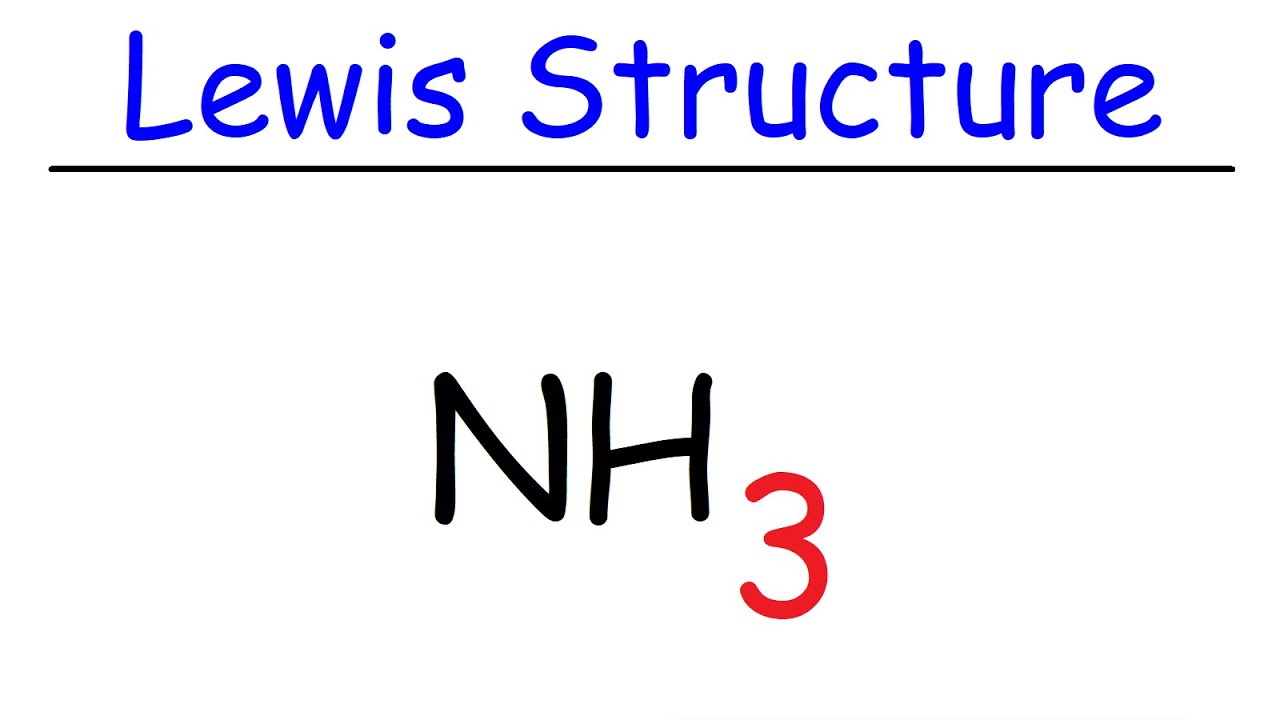
42 lewis dot diagram of ammonia
Which of the following is true regarding the Lewis diagram for ammoni a? (2 points) a. Ammonia contains three covalent bonds, one to each hydrogen atom, and the nitrogen atom has no lone pairs of electrons. b. Question: Draw the Lewis electron dot diagram for ammonia (NH3) on a sheet of scrap paper. You may consult the periodic table on Zoom to ... Schematic diagram showing the operation of an axial flow turbojet engine. Here, the compressor is again driven by the turbine, but the air flow remains parallel to the axis of thrust . Air intake. An intake, or tube, is needed in front of the compressor to help direct the incoming air smoothly into the moving compressor blades. Older engines had stationary vanes in front of the moving blades ...
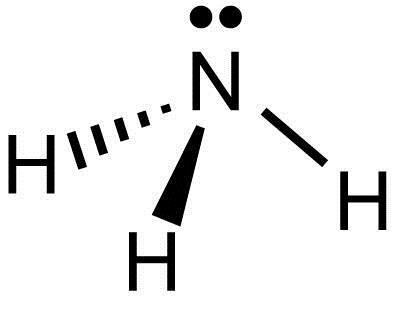
I quickly take you through how to draw the Lewis Structure of Ammonia, NH3. I also go over hybridization and bond angle.
Lewis dot diagram of ammonia
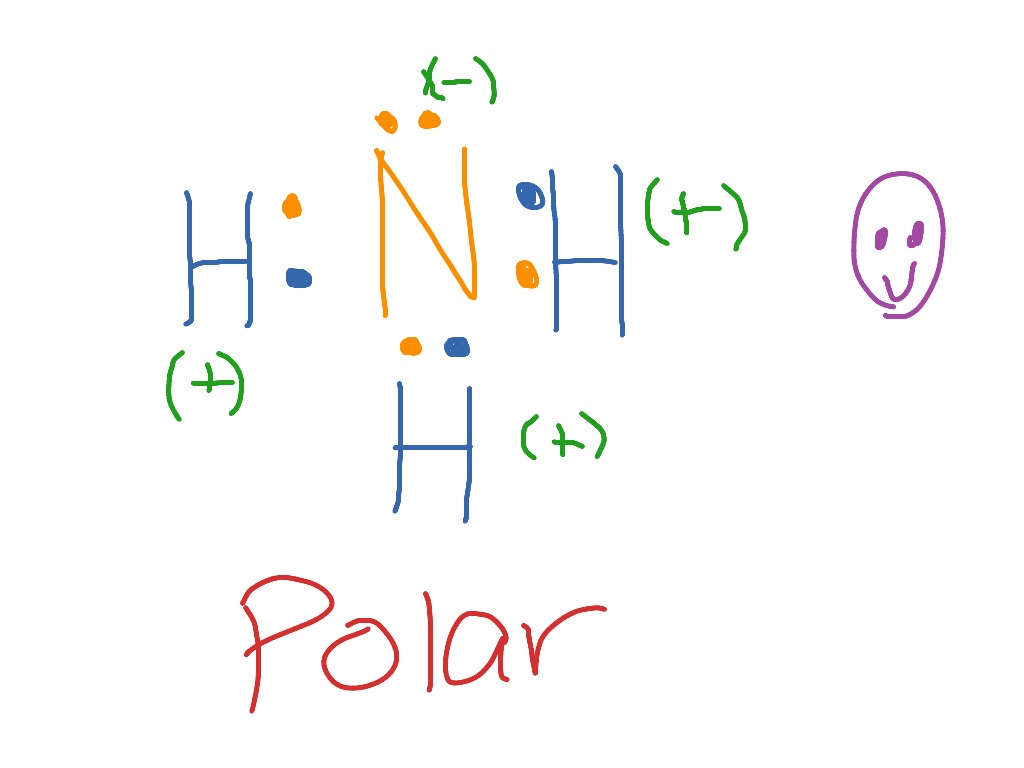
Oxidation state in metals. Many compounds with luster and electrical conductivity maintain a simple stoichiometric formula; such as the golden TiO, blue-black RuO 2 or coppery ReO 3, all of obvious oxidation state.Ultimately, however, the assignment of the free metallic electrons to one of the bonded atoms has its limits and leads to unusual oxidation states. Finally, students will draw a diagram and write an explanation of the apparent movement of stars using data from the graphs and class model. This lesson results from the ALEX Resource Gap Project. View Standards Standard(s): [MA2015] PRE (9-12) 29 : 29 ) (+) Use special triangles to determine geometrically the values of sine, cosine, and tangent for π / 3, π / 4, and π / 6, and use the unit ... 5 May 2018 — The Lewis structure of ammonia, NH3 , would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons ...1 answer · Have a look here... Explanation: The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone ...
Lewis dot diagram of ammonia. A step-by-step explanation of how to draw the NH3 Lewis Dot Structure (Ammonia).For the NH3 structure use the periodic table to find the total number of vale... Hey everyone, welcome to the Mentor Center! In today's video, I draw out the Lewis dot structure for NH3, commonly known as ammonia.👍 Like 📽️ Subscribe ... Lewis Structure Questions and Answers. Get help with your Lewis structure homework. Access the answers to hundreds of Lewis structure questions that are explained in a way that's easy for you to ... Carbon dioxide A Lewis dot diagram for carbon dioxide. Hydrogen and Lithium. However, many atoms below atomic number 20 often form compounds that do not follow the octet rule. For example, with the duet rule of the first principal energy level, the noble gas helium, He, has two electrons in its outer level. Since there is no 1p subshell, 1s is followed immediately by 2s, and thus level 1 can ...
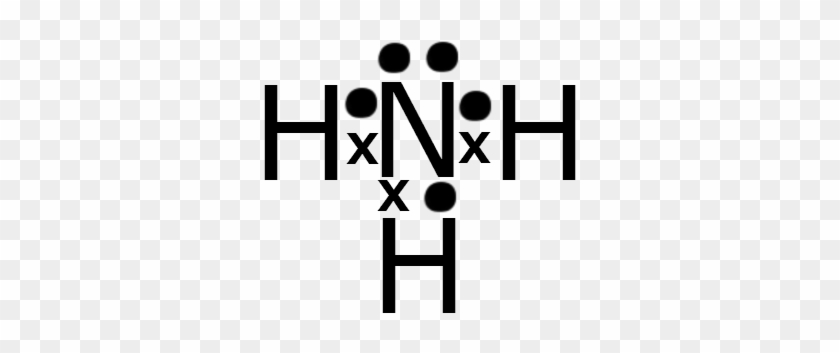
Craig Beals shows how to draw the Lewis Structure for Ammonia.This is a clip from the complete video: Covalent Bonding 2.1 - Drawing Lewis Structures Use information from step 4 and 5 to draw the lewis structure. Nitrogen goes in the centre. Lewis dot structure of ammonia. Alternatively a dot method can be used to draw the lewis structure of NH3. Calculate the total valence electrons in NH3 molecule. N=5,H=1x3=3 Total=8 Put Nitrogen in the center and three hydrogen atoms on the sides. Q. This is the correct dot diagram for sodium, group 1. Q. This is the correct dot diagram for nitrogen, group 15. Q. This is a correct dot diagram for neon, group 18. Q. This could be the dot diagram of. Mg, group 2. A simple method for drawing the Lewis structure for ammonia.
Ammonia (NH3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer. It also is a good example of a molecule with a ...25 Oct 2016 · Uploaded by Wayne Breslyn 06.11.2021 · Step 2: Now we will draw the Lewis dot structure of the compound. See the diagram below: Now you can see that the central atom here is Carbon because it is easy for Carbon to become stable as it is the least electronegative of all. However, hydrogen is the least electronegative but it cant be a central atom because it has only one spare electron. The molecular weight of Ammonia is 17 g/mol. It is a colourless alkaline gas. Starting with the Lewis dot structure of Ammonia, Nitrogen has 5 valence electrons ... 2; Dot Diagram Practice for Covalent Compounds (multiple copies) Ch. No Virtual Class - No Zoom Class. 5 H2O were heated to drive off all the water, the anhydrous salt CuSO4 would weigh 63. flushingschools. If these rigid segments are completely removed by hydrogenation (H 2 & Pt catalyst), the chains lose all constrainment, and the product is a low melting paraffin-like semisolid of little ...
06.11.2021 · Ph3 covalent compound name

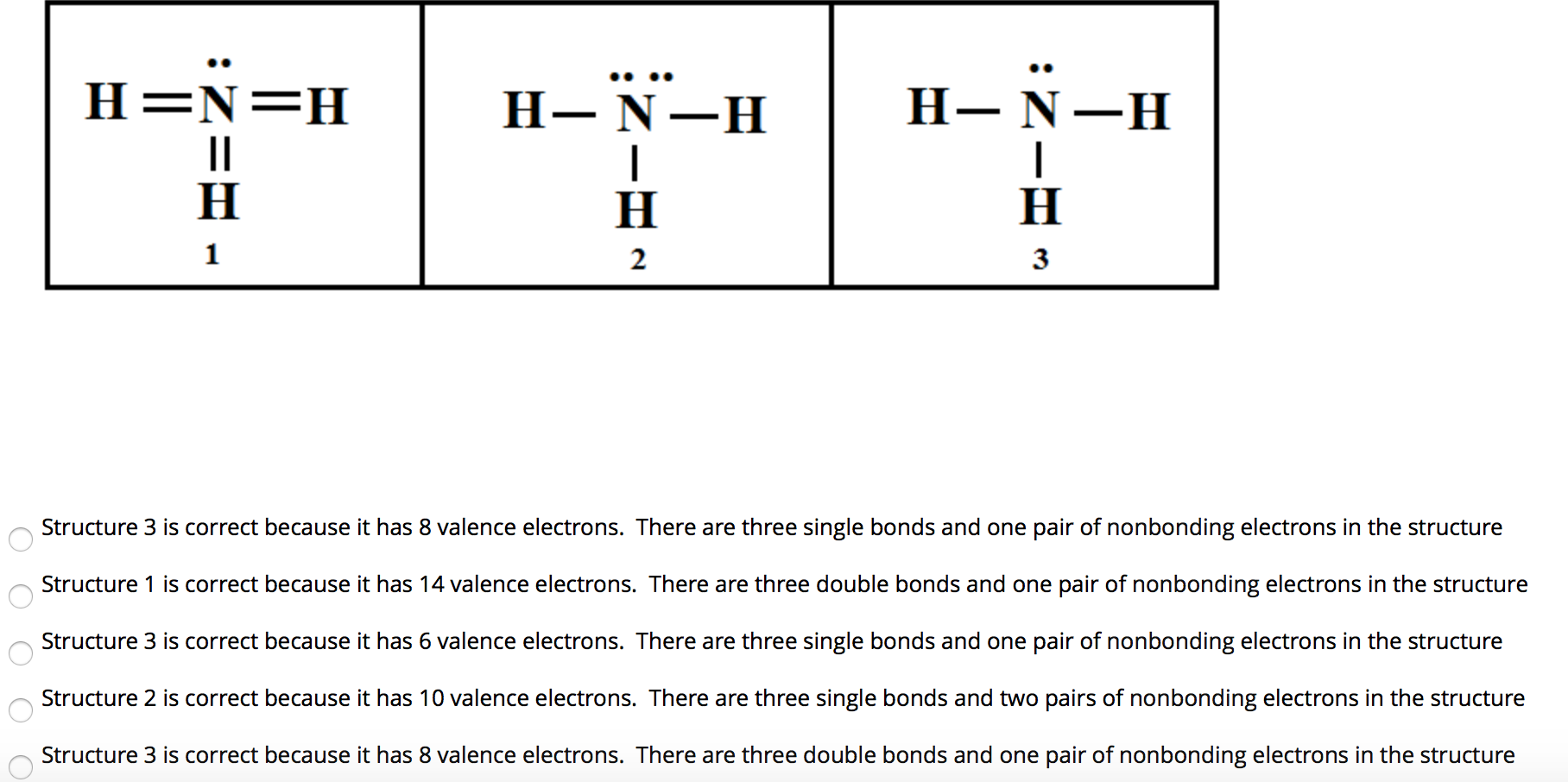
Which Of The Following Is The Correct Lewis Structure For Ammonia Nh3 Note Hydrogen And Nitrogen Brainly Com
So for Ammonium you need to identify the valence electrons for each of the elements. · Nitrogen has 5 | Hydrogen has 1 * 4 | 4 Being the number of Hydrogen's.3 answers · 2 votes: Electrons of N is shared by H to complete its duplet and elctrons of H is shared by N to ...
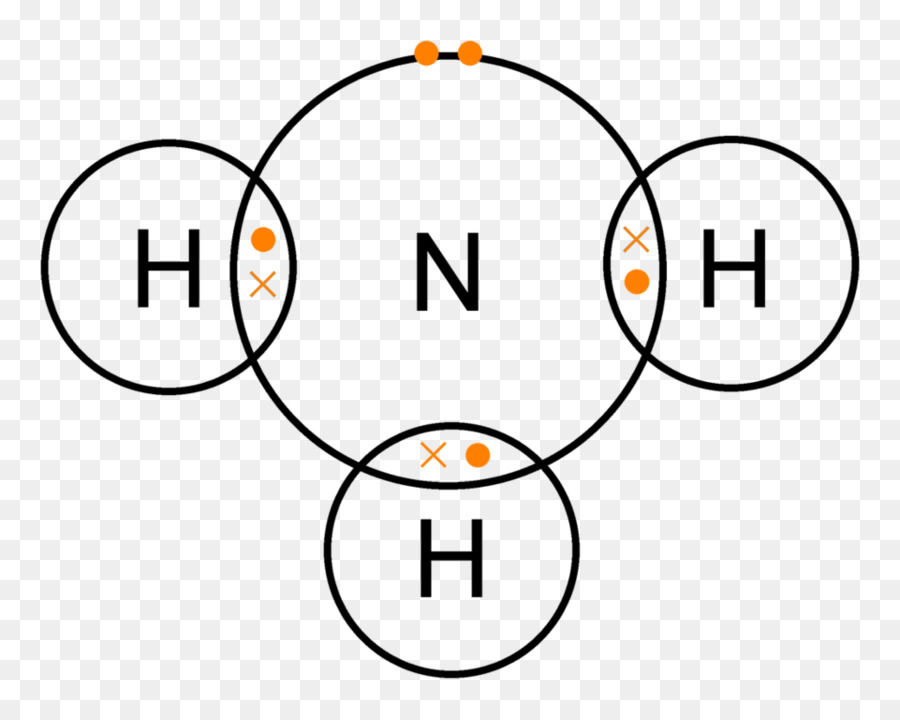
The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Electron Dot Structure of NH3 by Jeff Bradbury - February 17, - Lewis Electron Dot Structure for ammonia molecule NH3.
A video explanation of how to draw the Lewis Dot Structure for Ammonia, along with information about the compound including Formal Charges, Polarity, Hybrid ...
Ammonia lewis structure contains three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH3 can be drawn by using valence electrons of ...
5 May 2018 — The Lewis structure of ammonia, NH3 , would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons ...1 answer · Have a look here... Explanation: The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone ...
Finally, students will draw a diagram and write an explanation of the apparent movement of stars using data from the graphs and class model. This lesson results from the ALEX Resource Gap Project. View Standards Standard(s): [MA2015] PRE (9-12) 29 : 29 ) (+) Use special triangles to determine geometrically the values of sine, cosine, and tangent for π / 3, π / 4, and π / 6, and use the unit ...

A Molecule Of Ammonia Nh3 And A Molecule Of Methane Ch4 Each Have 4 Electron Domains A Draw Or Describe The Lewis Dot Structures For Each Of These Molecules B Explain Why
Oxidation state in metals. Many compounds with luster and electrical conductivity maintain a simple stoichiometric formula; such as the golden TiO, blue-black RuO 2 or coppery ReO 3, all of obvious oxidation state.Ultimately, however, the assignment of the free metallic electrons to one of the bonded atoms has its limits and leads to unusual oxidation states.

Ammonia 2d Dot Cross Dot Cross Diagram For Ammonia Png Image Transparent Png Free Download On Seekpng

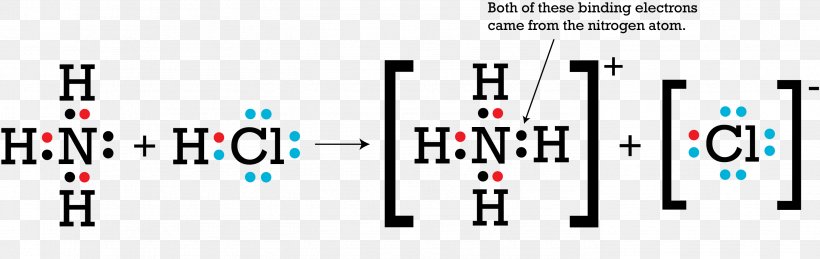
Best Answer Draw The Electron Dot Structure Of Ammonia Molecule And Show The Formation Of Ammonium Brainly In
Draw An Electron Dot Diagram To Show The Formation Of Ammonium Ion Atomic No N 7 And H 1 Sarthaks Econnect Largest Online Education Community



















0 Response to "42 lewis dot diagram of ammonia"
Post a Comment