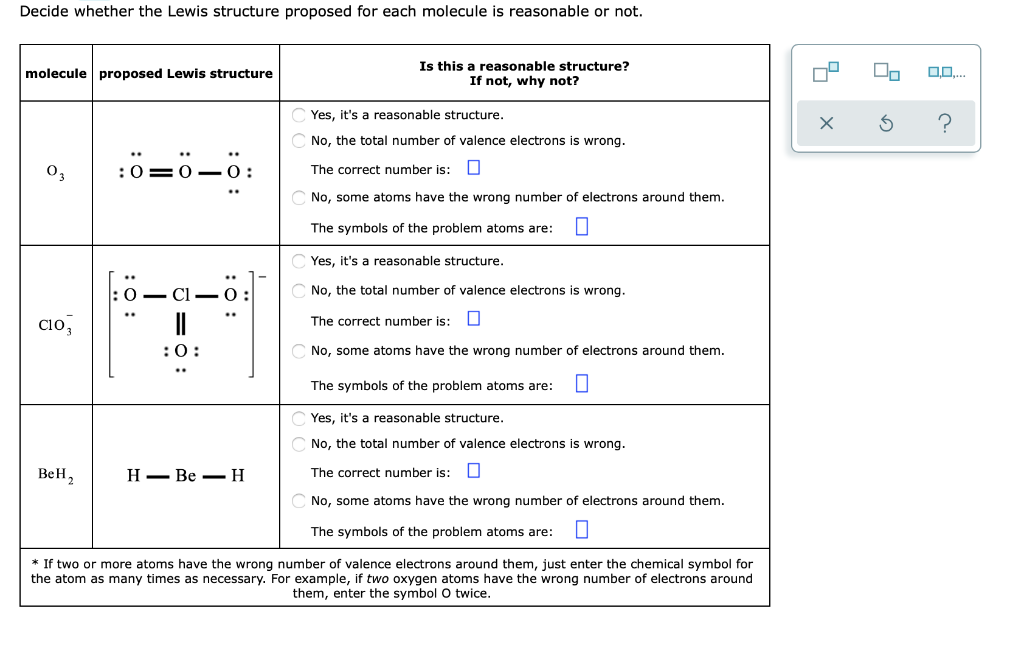
42 lewis dot diagram for o3
1. Start by drawing the Lewis Dot Structure for all using S=N-A For BF3: B= 3 ve F3= 21 ve A= 21 + 3= 24 B wants 8 F wants 8 N= 8 + 24= 32 S= 32-24= 8/2=4 bonds 2. Draw your Lewis structure adding in your dipole moments. Fluorine is the most electron negative atom so all electrons will be pulled towards your Fluorines making BF3 non polar. Review what a Lewis dot diagram is and discover how to draw a Lewis dot structural formula for compounds. Learn how to represent single, double and triple bonds with lines instead of dots. Also ...
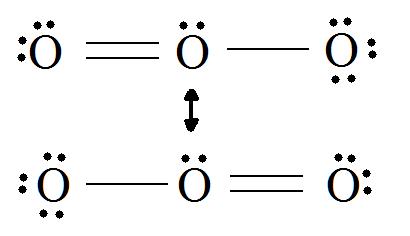
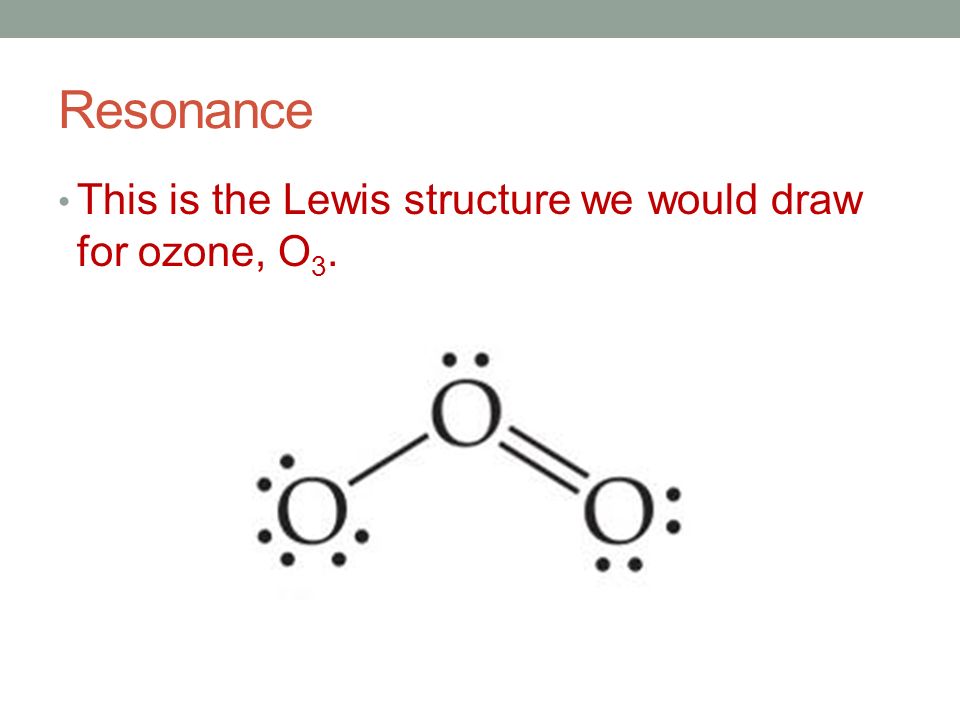
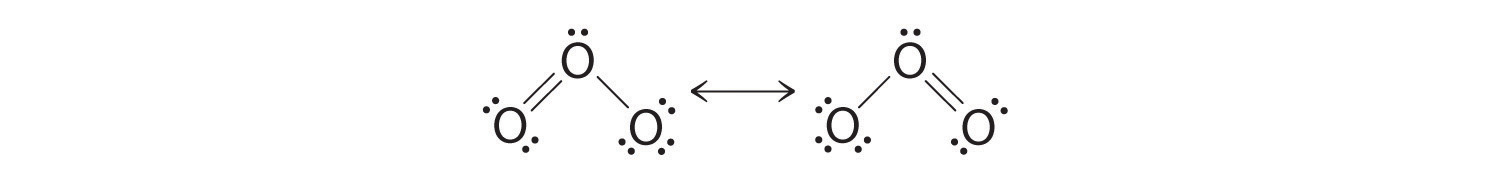
Lewis dot structure for O3. Wiki User. ∙ 2010-12-06 00:29:36. See Answer. Best Answer. Copy..:O=O: |:O:.. this would have a resonance structure too. 18 electrons total. 1 single bond. 1 double ...
Lewis dot diagram for o3
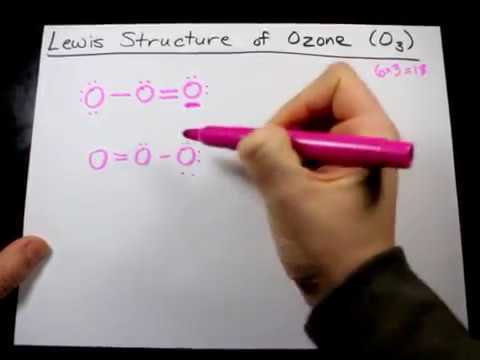
A step-by-step explanation of how to draw the O3 Lewis Dot Structure (Ozone).For the O3 structure use the periodic table to find the total number of valence ... Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, whether they exist as lone pairs or within bonds. Lewis dot diagrams for elements are a handy way of picturing valence electrons, and especially, what electrons are available to be shared in covalent bonds. The valence electrons are written as dots surrounding the symbol for the element: one dot is place on each side first, and when all four positions are filled, the remaining dots are paired ...
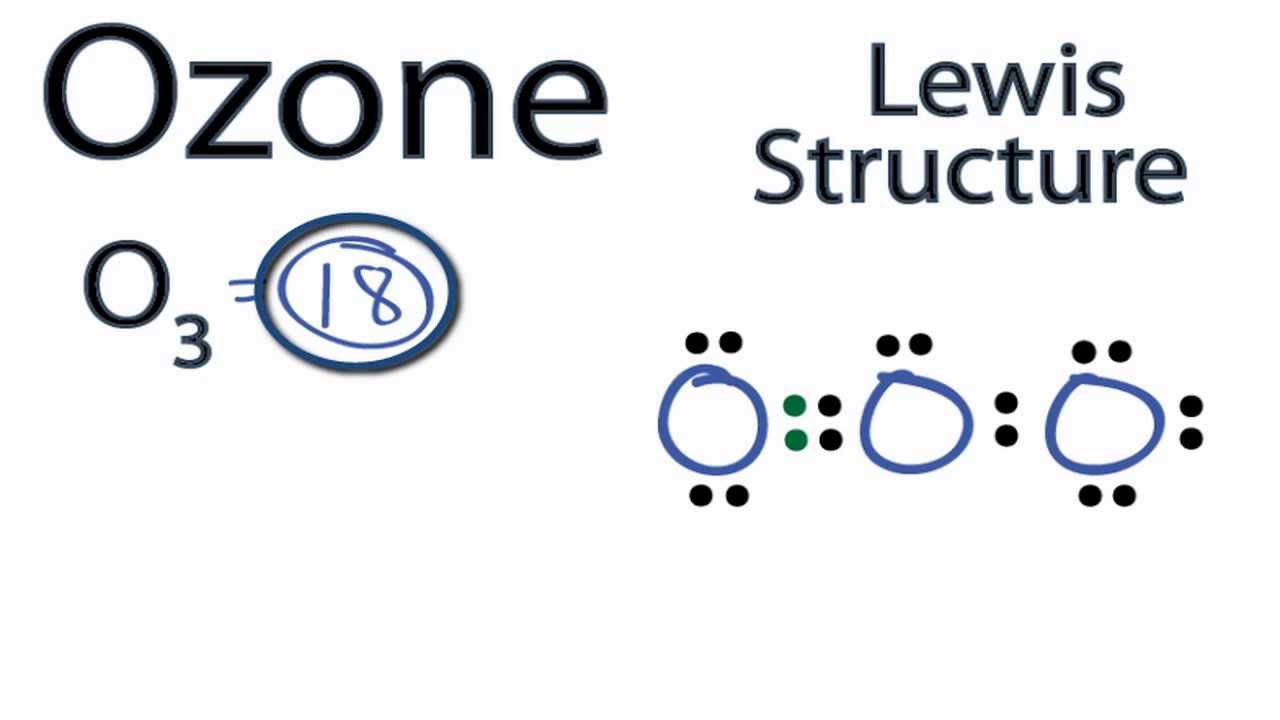
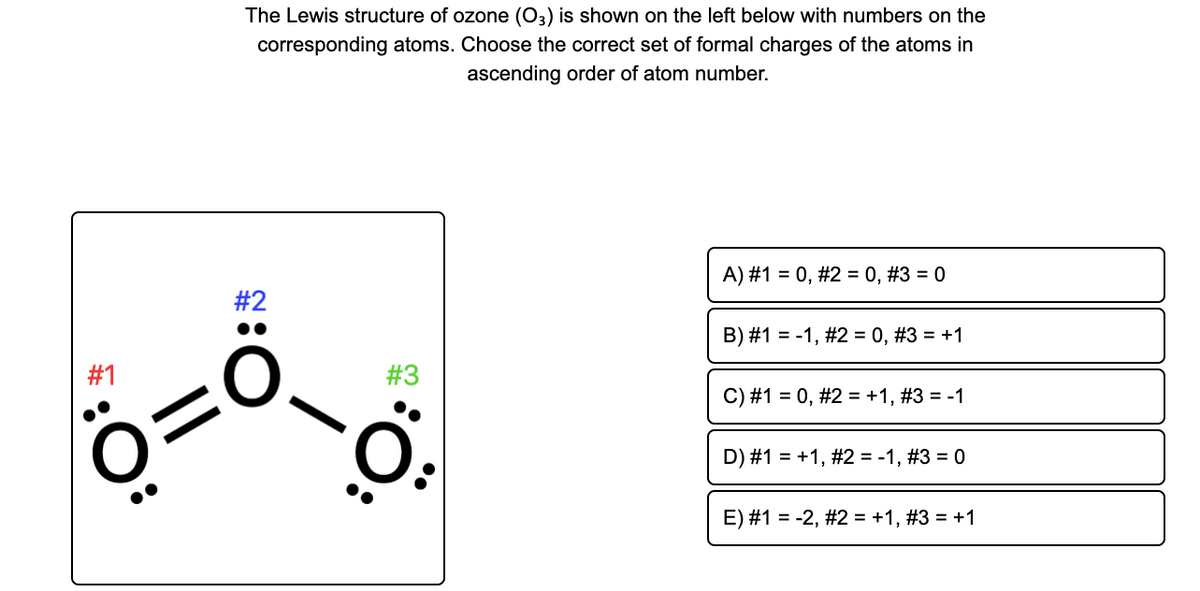
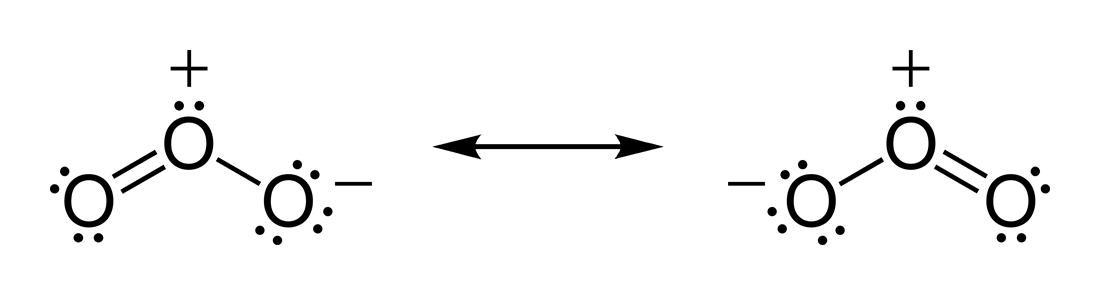
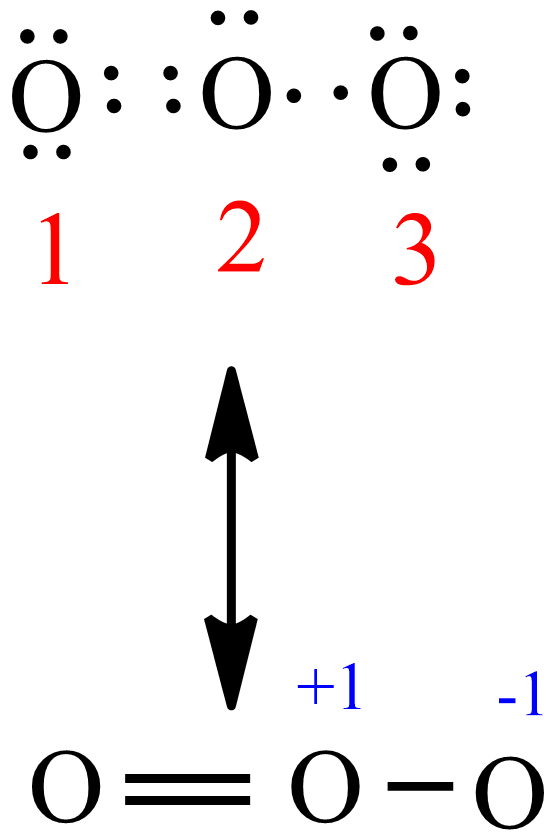
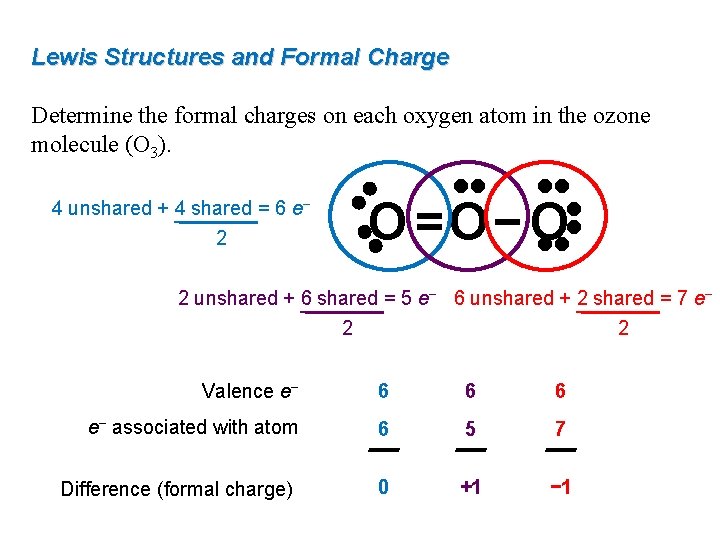
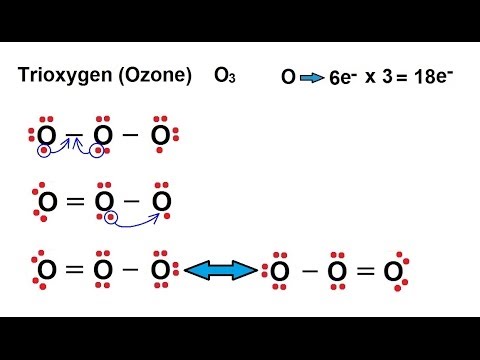
Lewis dot diagram for o3. Ozone (O3) is an allotrope of oxygen and contains three oxygen atoms. In the lewis structure of ozone, there are one double boond and one single bond. Also, there are charges in two oxygen atoms in O3 lewis structure. Draw the Lewis Dot Structure for O3? What is the total number of valence ELECTRONS? 18 valence electrons. Draw the Lewis Dot Structure for SO3^2-? What is the total number of valence ELECTRONS? 26 valence electrons. YOU MIGHT ALSO LIKE... 133 terms. Lewis Dots & Molecular Bonding. 66 terms. Lewis Structure of O3. Here, we will be dealing with ozone, the molecular formula is O3. The below discussion, therefore, will be based on finding out the Lewis Structure of O3. Ozone consists of three oxygen atoms. Oxygen belongs to group VI of the periodic table with an atomic no of 8. It thus has 6 valence electrons. Explanation: Simple VESPER requires that we distribute 3 ×6 = 18 valence electrons across 3 centres: O = O+ − O−. From the left, O1, has TWO lone pairs; O2 has ONE lone pairs; and O3 has THREE lone pairs. And thus the formal charge of each oxygen atom ( 8e−,7e−,9e−) is 0, + 1, −1 respectively. Because there are THREE regions of ...
A Lewis structure is a graphic representation of the electron distribution around atoms. The reason for learning to draw Lewis structures is to predict the number and type of bonds that may be formed around an atom. A Lewis structure also helps to make a prediction about the geometry of a molecule. Lewis dot structures are diagrams of atoms and valence electrons as they are arranged in a molecule. Review Lewis dot structures and its steps, then examine resonance and resonance structures of ... Click here👆to get an answer to your question ️ Draw the Lewis dot structure of the following. CO3^2 - , HClO4 , HNO3 Give the lewis electron dot structure for no2â€. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. Structure s structure sure if your structure is correct, do a formal charge check. Eribulin may also cause low levels of infection. Also, there are charges in two oxygen atoms in o3 lewis structure.
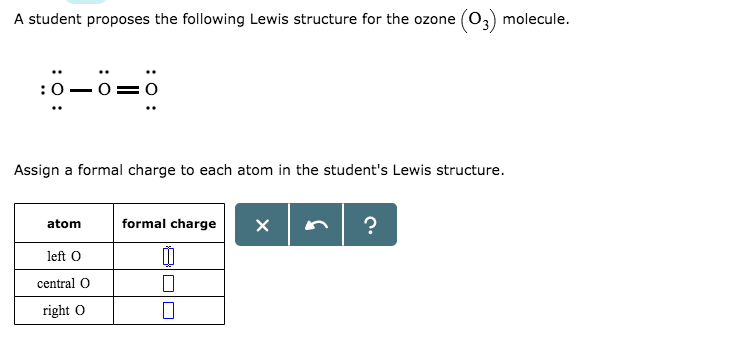
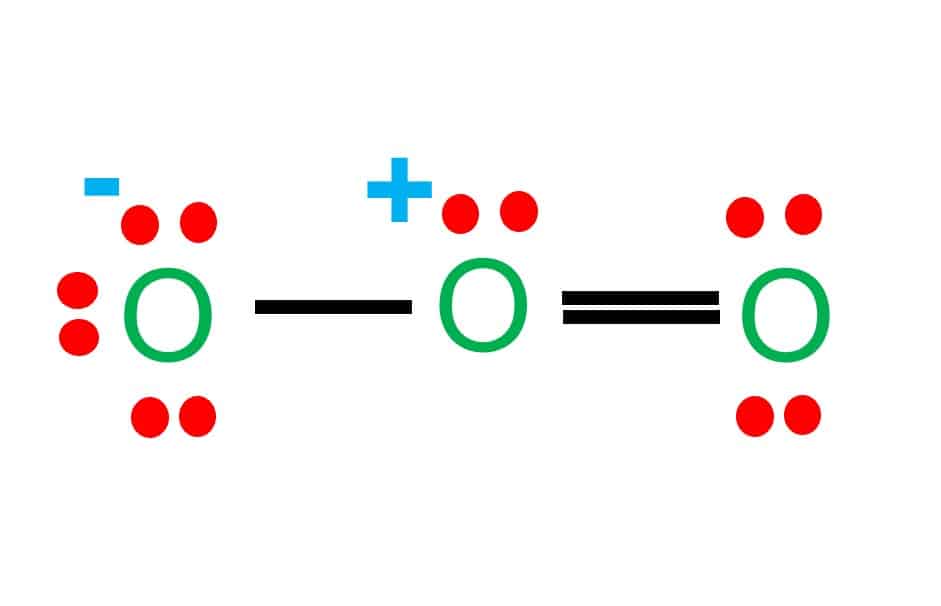
The formal charge on each O- atom of O3 molecule is given as,The Lewis structure of O3 may be drawn as:The atoms have been numbered as 1, 2 and 3. Formal charge (F.C.) on end O-atom numbered 1. Formal charge (F.C.)on central O-atom numbered. Formal charge (F.C.) on end O-atom numbered 3.Hence, we represent O3 along with the formal charges as follows: Get the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha. I was wondering why the Lewis structure for O3 is O-O=O ( where the Formal charge from left to right is -1,+1,0) (3 e- pairs. Consider the case of ozone O3 Lewis electron dot structures: 2 π electrons (pi electrons) in O3 and so 1 double bond must be added to the structure of Step 1.How to Draw a Lewis Structure Find the Total Number of ... 2. Draw the Lewis dot structures for each of the following molecules: a. H 2 S c. SO 3 b. CH 2 Br 2 d. HCN 3. Draw the Lewis dot structure for each of the following polyatomic ions: a. NH 4 + c. PO 4 -3 b. NO 3 - d. CO 3 2- 4. For the following molecules or ions (where the central atom is underlined): i. Draw the Electron dot structure. ii.
The central atom in the Lewis structure will have a charge of +1 and the atom forming a single bond will have -1 charge. O3 Hybridization Hybridization in chemistry means the hybridising of two or more atomic levels of the same or different energies to combine and give a new orbital.
Answer (1 of 3): The Lewis structure of ozone (O3) 1. Sum of valence electrons = (6*3) = 18 2. Drawing the bond connectivities: 3. Complete the octets of the atoms bonded to the central atom: 4. Place any leftover electrons (18-16 = 2) on the central atom: 5. Does the central atom have a...
12+ O3 Lewis Structure. In general, only c, n, o, and s form multiple (double and triple) bonds. Lewis structure helps to know the number of valence electrons in the molecule. Peroxides, superoxides, molecular oxygen, o2, and ozone, o3. The typical lewis structure of ozone depicts formal charge separation.
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds.
How to draw the Lewis dot diagram for ozone. How to draw the Lewis dot diagram for ozone.
• (3c) ·Lewis dot diagrams are used to represent valence electrons in an element. Structural formulas show the arrangements of atoms and bonds in a molecule and are represented by Lewis dot structures. • Draw Lewis dot diagrams to represent valence electrons in elements and draw Lewis dot structures to show covalent bonding.
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
The Lewis Dot Structure for O2. Created by MakeTheBrainHappy. This is the Lewis Dot Structure for O2, commonly referred to as oxygen gas. Due to oxygen's high electronegativity (affinity for electrons), the pure element is nearly exclusively found in either this state or ozone (O3 - a distinct lewis structure for another post).
what is the Lewis electron dot structures for ozone O3, pi and, what is the Lewis electron dot structures for carbonates CO3-2, Simple Procedure for writing Lewis Structures for O3 and carbonate ion, co32-, o3 lewis structure, lewis structure, lewis dot structure, CO3-2 lewis structure, O3 lewis structure, lewis dot diagram, electron dot diagram, Chemistry Net, chemistry tutorial on Lewis ...
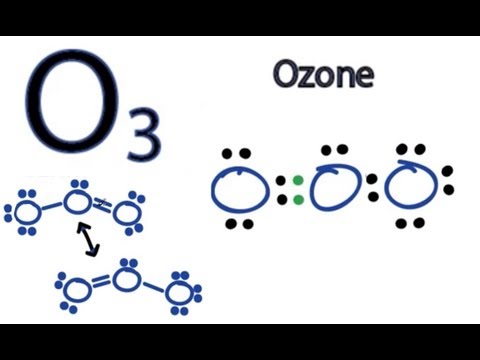
The Lewis Structure of Ozone has:* three oxygen atoms in a row. It is not a ring, although that might be tempting.* two resonance structures* a lone pair on...
Drawing the Lewis Structure for O 3. Viewing Notes: For the Lewis Structure for O 3 it is possible to draw it two different says (slightly different, but still important). These are called resonance structures. O 3 (Ozone) is important in the upper atomsphere since it blocks UV light that can be harmful to humans (for example: causing skin cancer).; Be sure that you don't use more than the 18 ...
Lewis dot diagrams for elements are a handy way of picturing valence electrons, and especially, what electrons are available to be shared in covalent bonds. The valence electrons are written as dots surrounding the symbol for the element: one dot is place on each side first, and when all four positions are filled, the remaining dots are paired ...
Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, whether they exist as lone pairs or within bonds.
A step-by-step explanation of how to draw the O3 Lewis Dot Structure (Ozone).For the O3 structure use the periodic table to find the total number of valence ...




















0 Response to "42 lewis dot diagram for o3"
Post a Comment