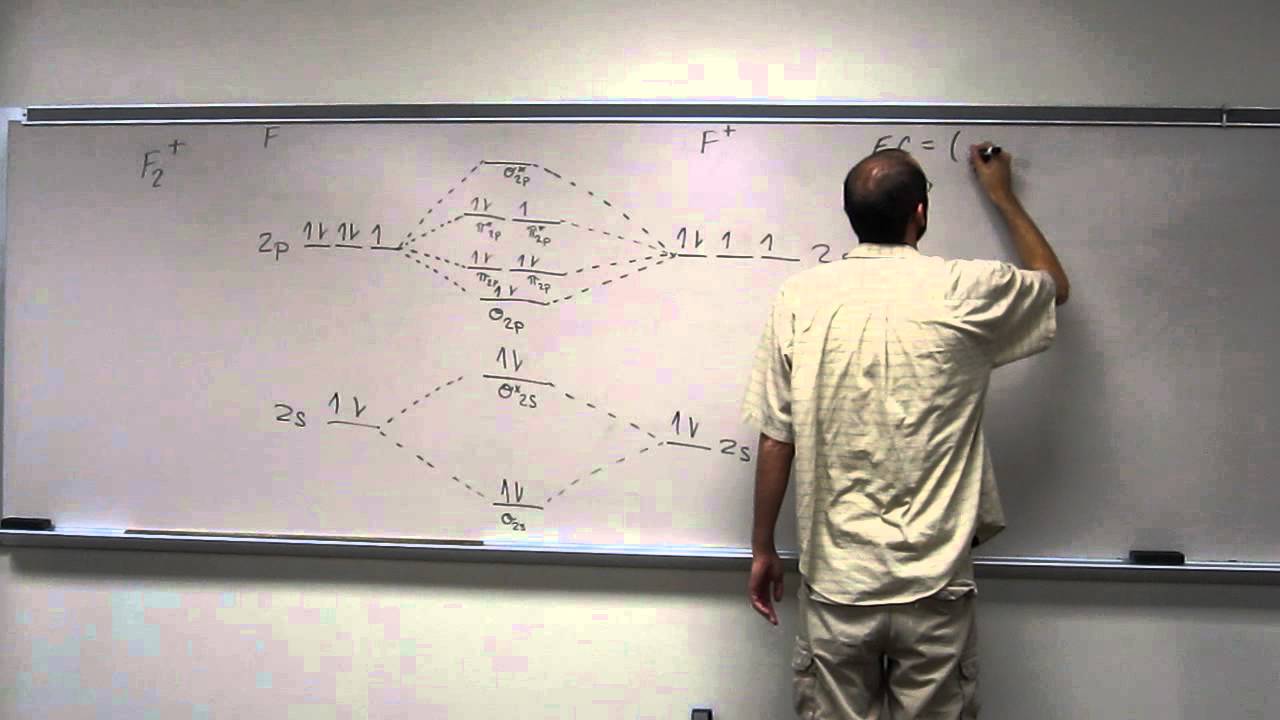
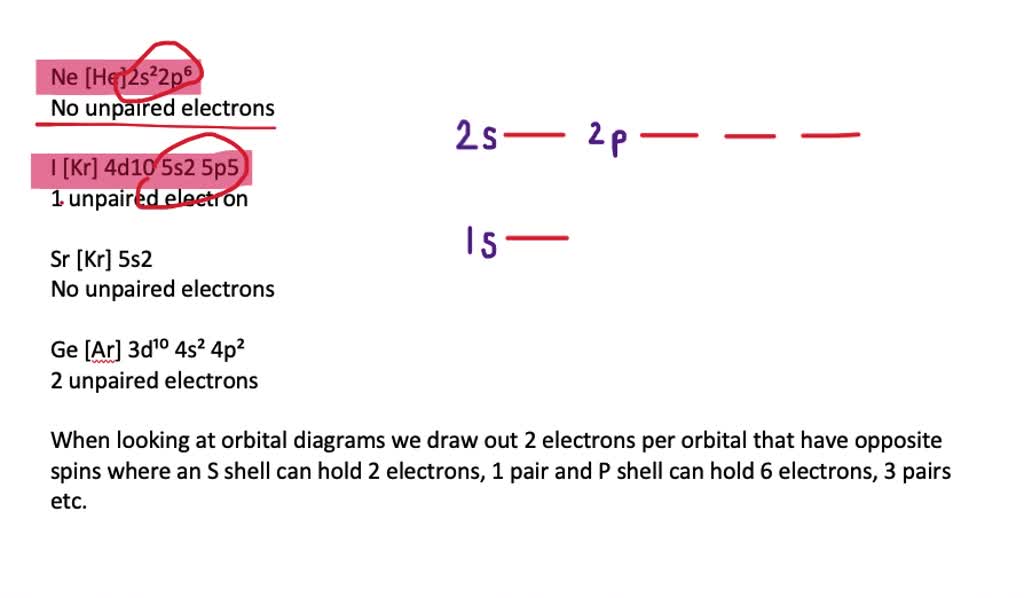
42 f2+ molecular orbital diagram
21.11.2021 · The molecular orbital diagram of ethane would be: The molecular orbital is formed from the combination of atomic orbitals, which must have nearly the same energy and are symmetrical about the molecular axis. To understand the MO diagram of … 22.11.2021 · For the PF4- Lewis structure use the periodic table to find the. In the Lewis structure of PF4-there are a total of 34 valence electrons. Hybridization in the Best Lewis Structure · 1. A bonding orbital for P1-F2 with 1.9546 electrons __has 9.66% P 1 character in a s0. · 2. A bonding orbital for P1- …
Molecular orbital diagram for f2. A fundamental principle of these theories is that as atoms bond to form molecules a certain number of atomic orbitals combine to form the same number of. C would this ion exist. To further demonstrate the consistency of the lewis structures with mo. For the ion f2.

F2+ molecular orbital diagram
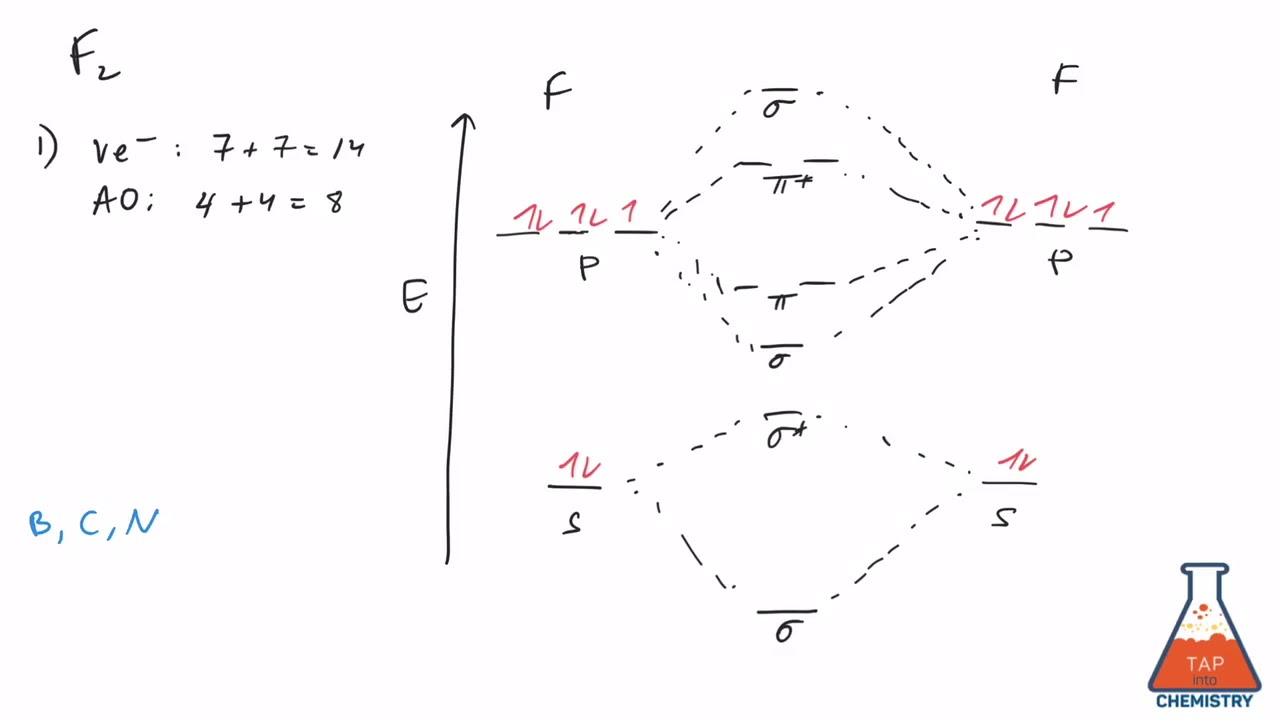
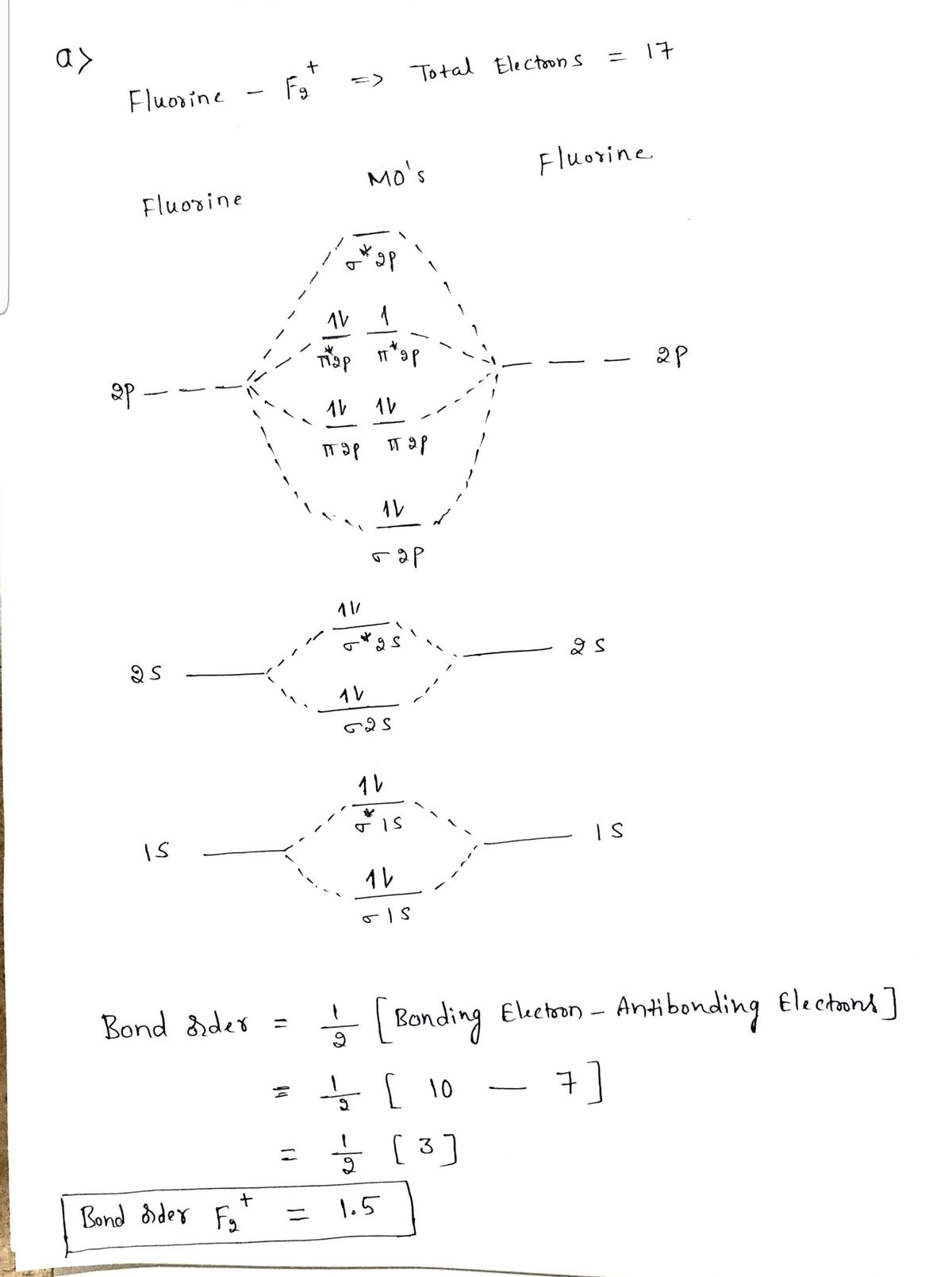
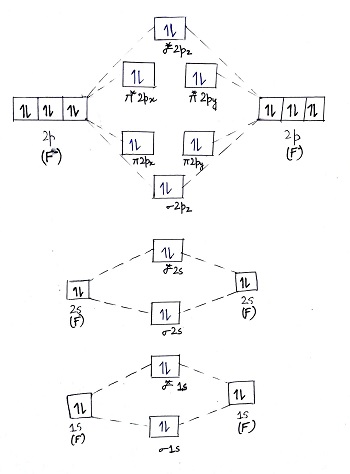
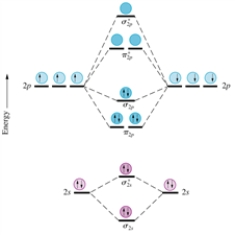
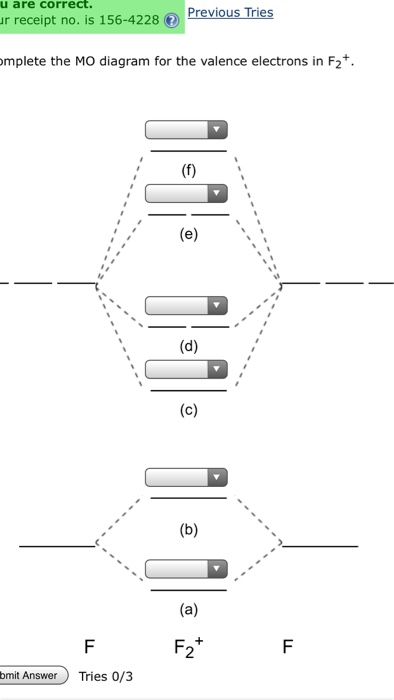
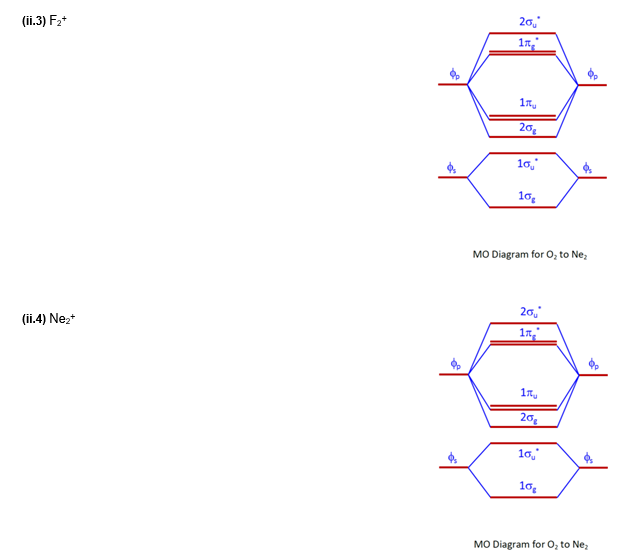
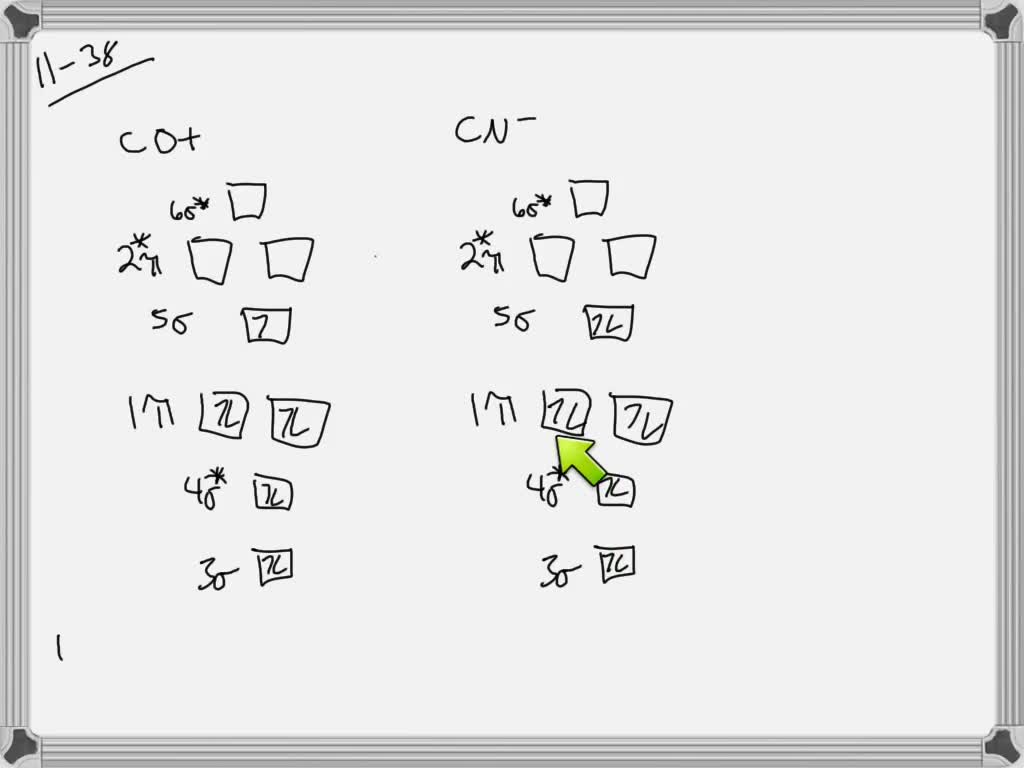
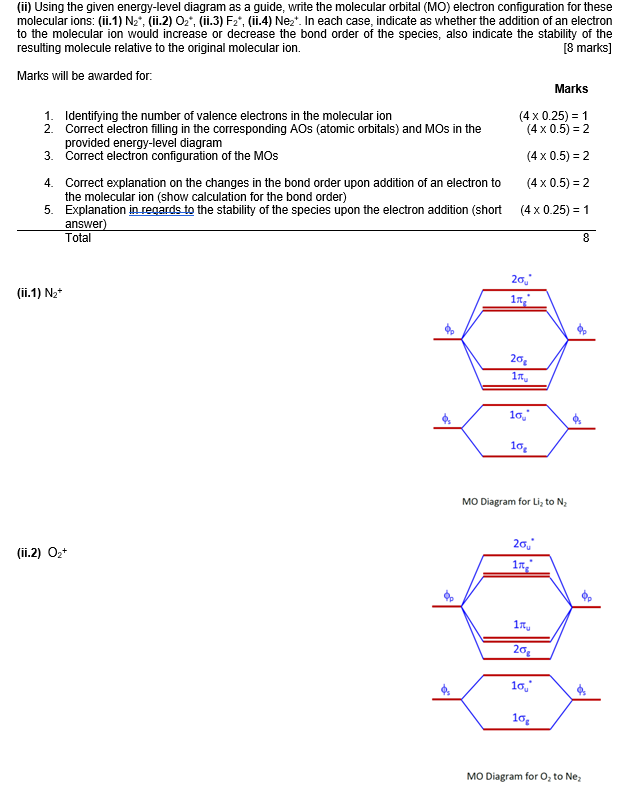
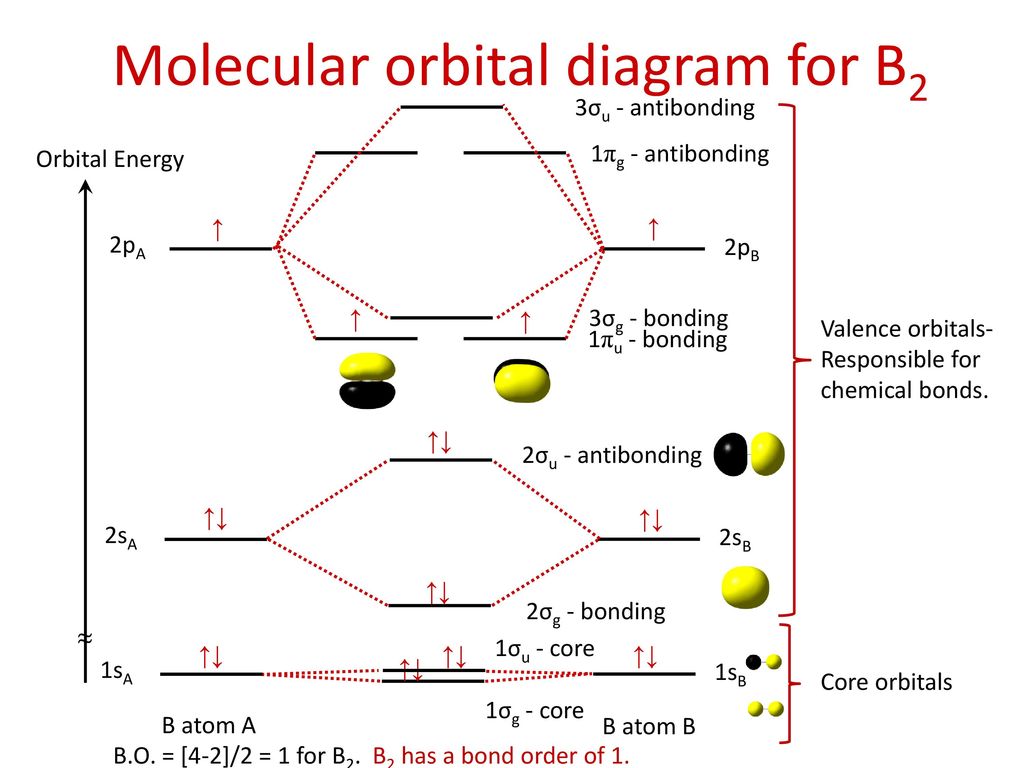
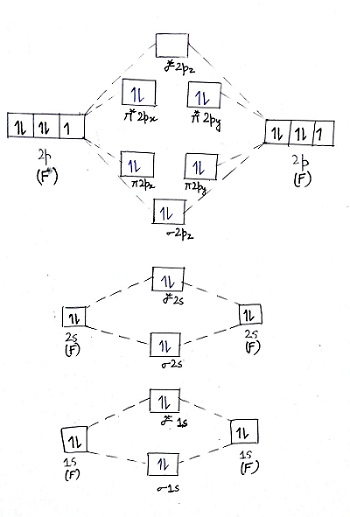
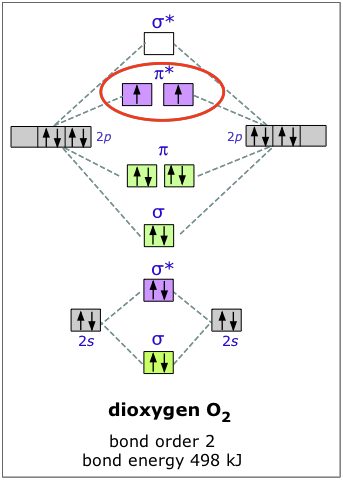
Aug 15, 2020 · The two electrons in the p* 2p 2 orbitals have the same spin, and they are responsible for the paramagnetism of oxygen. As an exercise, please fill electrons in the molecular orbitals of a relative energy level diagram to derive and confirm the above conclusion as well as the conclusion regarding the \(\ce{F2}\) molecule. The valence molecular orbital diagram for the cation F2+ is shown. Which of the following options correctly interpret this diagram? Select all that apply.-The molecular orbital bond order is equal to 3/2-F2+ has a stronger bond than F2. TikZ-Feynman is a LaTeX package allowing Feynman diagrams to be easily generated within LaTeX with minimal user instructions and without the need of external programs.It builds upon the TikZ package and its graph drawing algorithms in order to automate the placement of many vertices.TikZ-Feynman still allows fine-tuned placement of vertices so that even complex diagrams can be generated with ease.
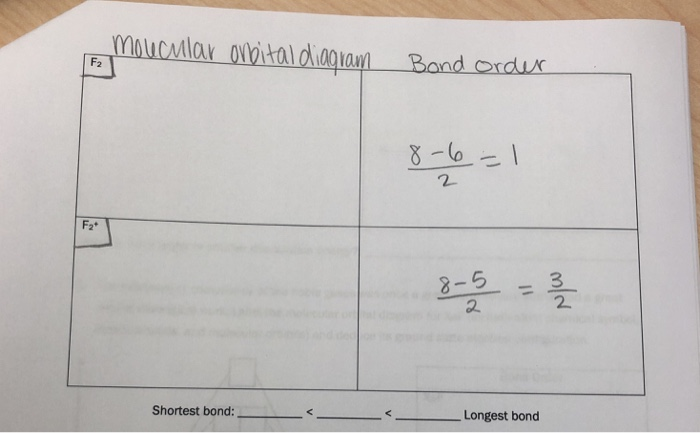
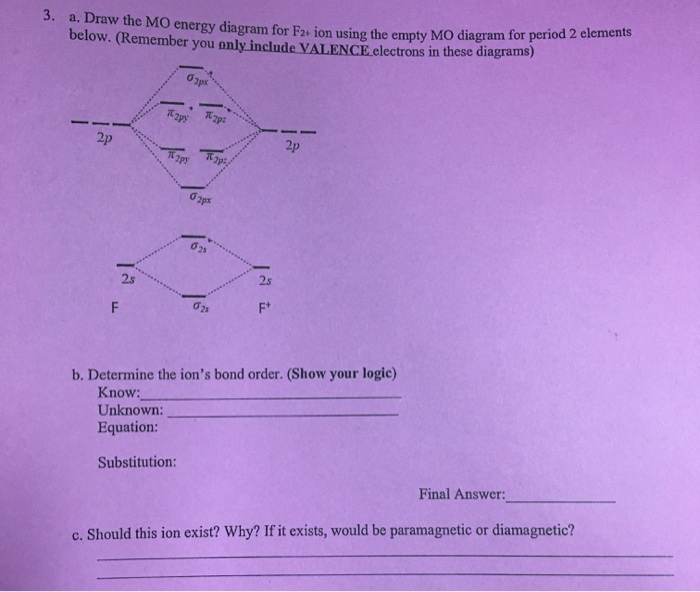
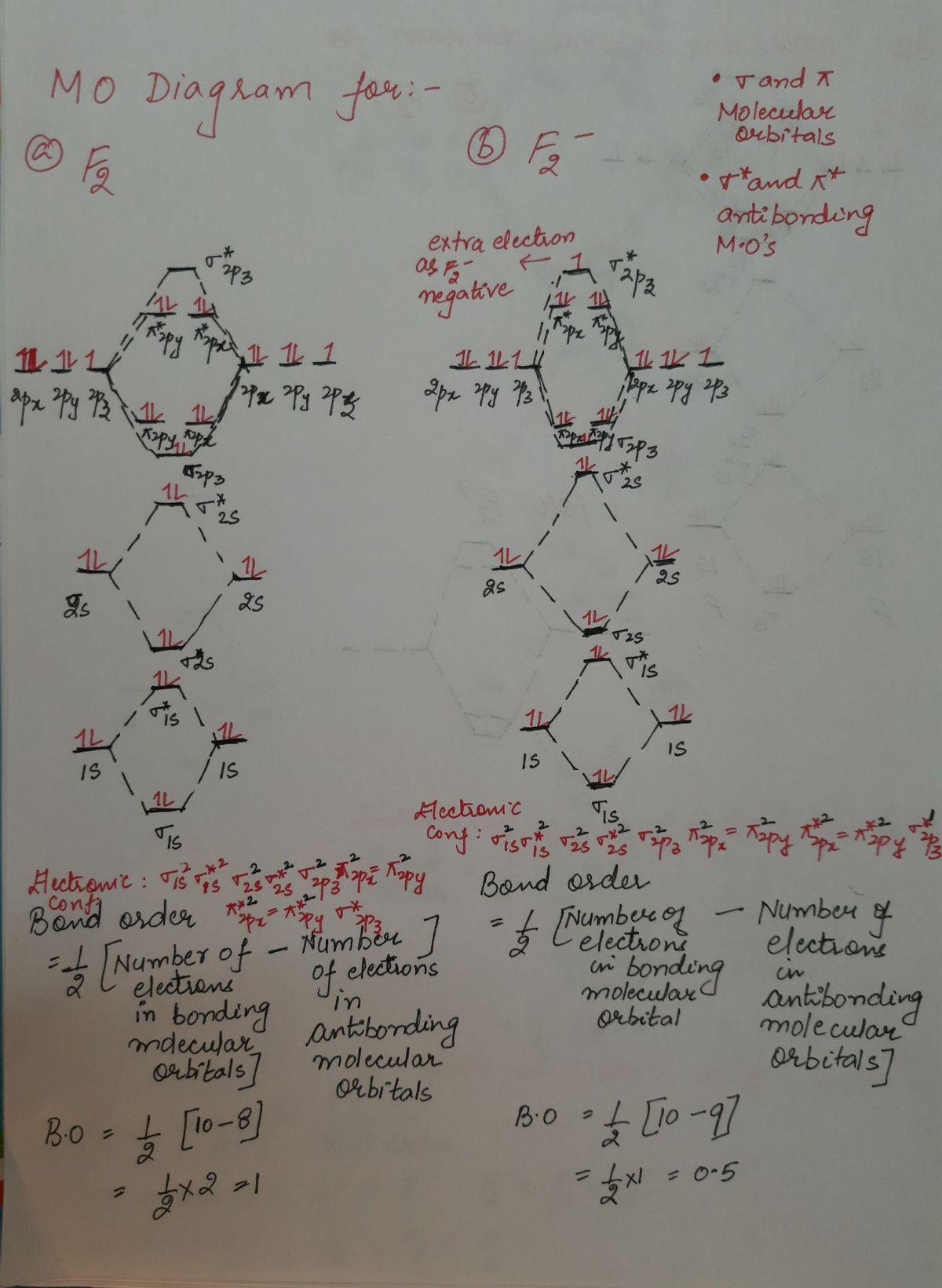
F2+ molecular orbital diagram. Free PDF download of Important Questions for CBSE Class 11 Chemistry Chapter 4 - Chemical Bonding and Molecular Structure prepared by expert Chemistry teachers from latest edition of CBSE(NCERT) books. Register online for Chemistry tuition on … The Lewis theory of chemical bonding helps us visualize the arrangement of atoms—how they are attached or bonded—in molecules. The valence electrons in each atom are the ones that participate in the bonding, and hence they are the only ones displayed in the Lewis structures. It is to be noted though that this theory about the electronic structure is quite primitive and most limited. In a typical Lewis structure, each valence electron is represented as a dot, and a covalent bond between two atoms (formed as a result of sharing of two electrons) is represented as a line. Several atoms tend to seek eight electrons in their valence shell through chemical bonding; this is referred to as the octet rule and is reflected in the Lewis structure of a molecule. Hydrogen is an exception, though; it seeks a duplet, not octet, because it has only one electron in its K shell, and thus needs only one more to achieve the maximum capacity of K shell. Noble gases already have completely filled valance... Sep 30, 2017 · A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. Molecular orbital diagram for f2. The other molecular orbital produced s h h shows a decrease in electron density between the nuclei reaching a value of zero at the midpoint between the nuclei where there is a nodal plane. When two fluorine atoms bond, the sigma(2p) bonding molecular orbitals are lower in energy than the pi(2p) bonding orbitals.F2(2+) has a bond order of 2, so ...
This video is about MO Diagram #2 - F2 Molecular orbital diagram for ne2 2 . Molecular orbital diagram for ne2 2 Molecular orbital diagram for ne2 2 ... The valence molecular orbital diagram for the cation f2+ is shown. which of the following options correctly interpret this diagram? * F2+ has a stronger bond than F2 *The olecular orbital bond order is equal to 3/2. Sigma Bonds vs. Pi Bonds. Sigma bond allows free rotation about the bond axis. 3.11.2021 · Molecular Orbital Diagram for Hydrogen For multi-electron atoms, draw the diagram only for the valence electrons. The formula for determining the bond order considers the valence electrons in …
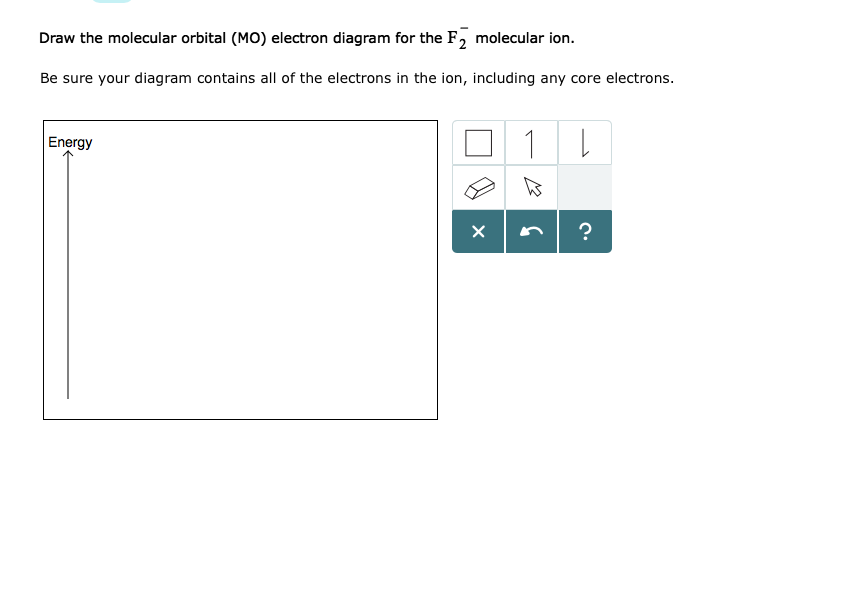
Answer (1 of 4): The atomic number of fluorine is 9, so a (neutral) F2 molecule has a total of 18 electron, or 14 valence electrons (excluding the four 1s electrons). The (F2)- ion has one more valence electron, or 15. The orbital diagram for a diatomic molecule is To find the bond order, add th... TikZ-Feynman is a LaTeX package allowing Feynman diagrams to be easily generated within LaTeX with minimal user instructions and without the need of external programs.It builds upon the TikZ package and its graph drawing algorithms in order to automate the placement of many vertices.TikZ-Feynman still allows fine-tuned placement of vertices so that even complex diagrams can be generated with ease. The valence molecular orbital diagram for the cation F2+ is shown. Which of the following options correctly interpret this diagram? Select all that apply.-The molecular orbital bond order is equal to 3/2-F2+ has a stronger bond than F2. Aug 15, 2020 · The two electrons in the p* 2p 2 orbitals have the same spin, and they are responsible for the paramagnetism of oxygen. As an exercise, please fill electrons in the molecular orbitals of a relative energy level diagram to derive and confirm the above conclusion as well as the conclusion regarding the \(\ce{F2}\) molecule.
















0 Response to "42 f2+ molecular orbital diagram"
Post a Comment