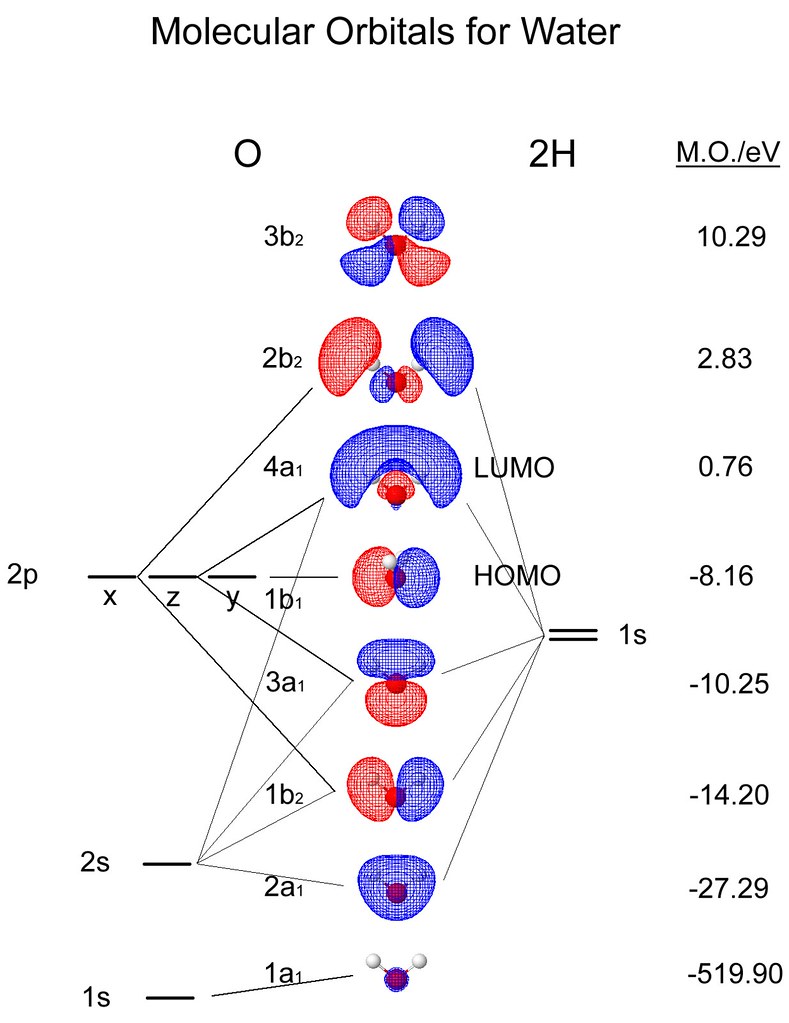
41 molecular orbital diagram for h2o
Construct a molecular orbital diagram. Be sure to do the following Draw an energy axiS List the Hls SALCs on the right hand side (the SALCs are the MOs for H2) List ALL of the valence atomic orbitals of the central atom on the left hand side Use the character table to assign the symmetry of the valence... Figure 9. The molecular orbital energy diagram predicts that H2 will be a stable molecule with lower energy than the separated atoms. Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule.
Learn how to draw a Molecular Orbital diagram. So rather the question should be "What is the bond order of oxygen in H2O2". (Am telling you from the homogenous system point of view where bond between similar atoms are considered… for higher study you can also learn MOT for heterogeneous...
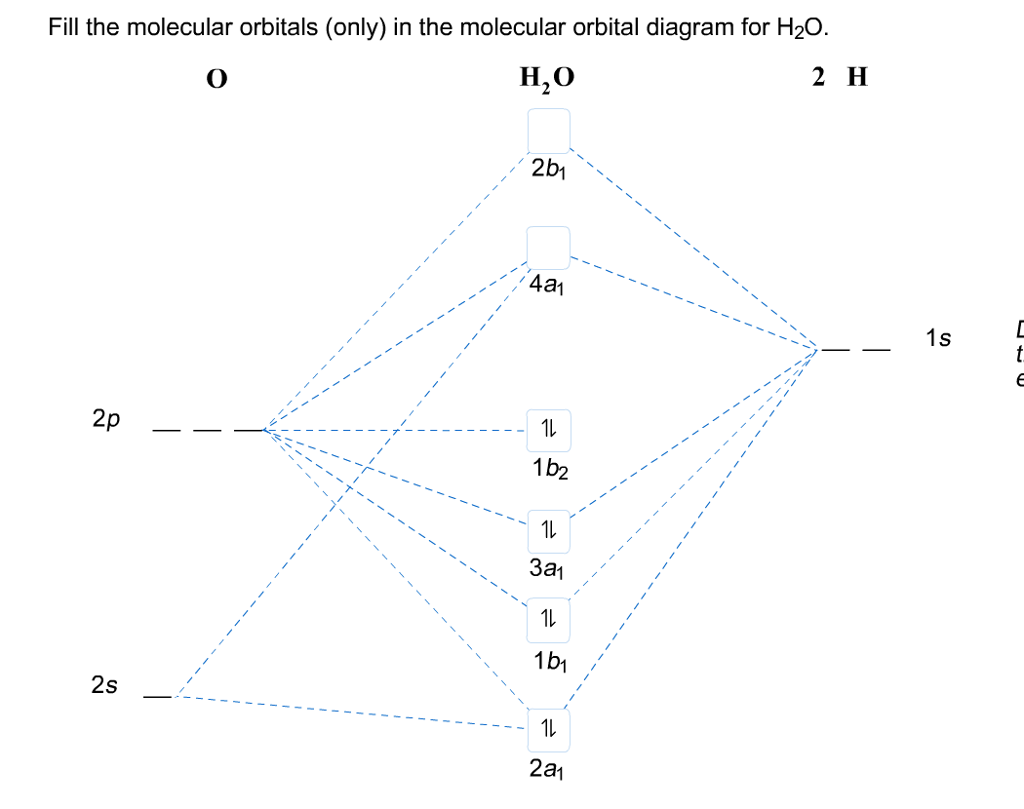
Molecular orbital diagram for h2o
- MO diagrams for Inorganic complexes. • It is a waste of both the lecturers and students time if the tutorial to ends up being a lecture covering questions. 5. An introduction to Molecular Orbital Theory. Molecular orbital calculations indicate, however, that for O2, F2, and hypothetical Ne2 molecules, the 2p orbital is lower in energy than the 2p orbitals The solid lines represent the relative energies of the indicated atomic and molecular orbitals. (a) The diagram for H2, He2, Li2, Be2, B2, C2, and N2... File:H2O-MO-Diagram.svg. From Wikimedia Commons, the free media repository. Jump to navigation Jump to search. Added orbital diagrams for molecular orbitals.
Molecular orbital diagram for h2o. Molecular orbital theory for diatomic molecules. Thus we can draw ENERGY LEVEL DIAGRAM for m.o.'s of H2 Molecular Orbital Energy Level Diagram for a Heteronuclear Diatomic. Furthermore, the molecular orbital diagram helps with determining how two sigma bonds have been formed and the effect of the lone pairs on the structure. The Lewis structure of the triatomic H2O molecule shows two single sigma bonds between the oxygen atom and the hydrogen atoms. This molecular orbital model can be used to explain why He2 molecules don't exist. Combining a pair of helium atoms with 1s2 electron configurations would The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the... Molecular orbital diagram - Wikipedia Molecular orbital theory (MO theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. It also explains the bonding in a number of other molecules, such as violations of the octet rule and more molecules...
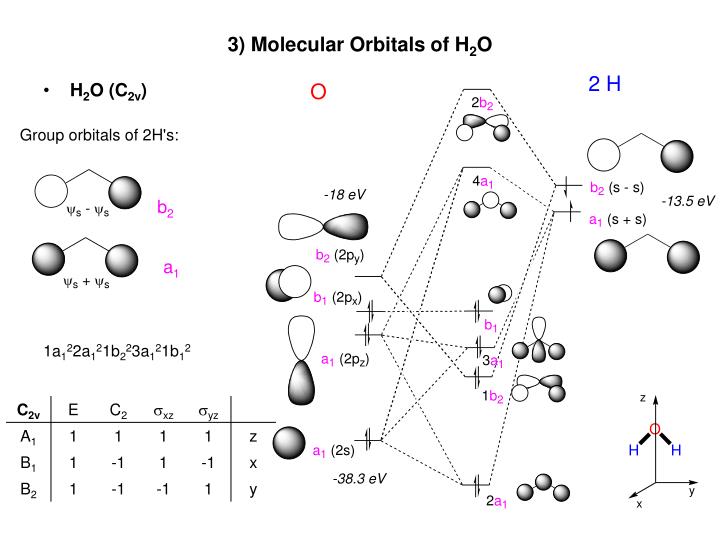
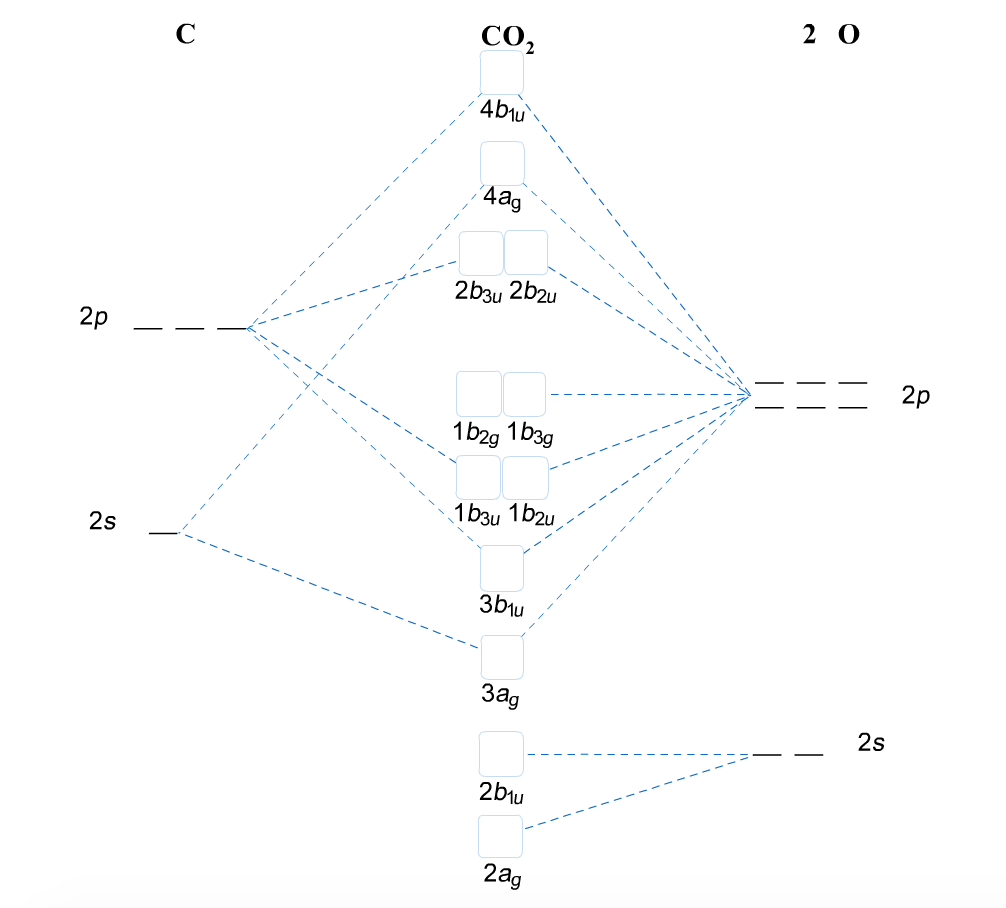
The molecular orbital energy- level diagram that results is constructed by putting the molecular orbitals in order of increasing number of internuclear As a first illustration of this procedure, consider the structures of the diatomic molecules formed by the period-2 elements (such as N2 and O2). Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons. Assembly of the molecular orbital diagram for water C2y point group) using symmetry-adapted orbitals on the ligands and the central atom/ orbitals. The H-C-H bond angle in methane is 109.5°. The H-O-H bond angle of water is close to this number but the H-S-H bond angle of H2S is near 90°... With knowledge of both orbital symmetries and energies, we can construct the molecular orbital diagram. The valence orbitals of oxygen go on one side of In the previous examples shown for the molecular orbital diagrams of the bifluoride anion and carbon dioxide, we discussed differences in...
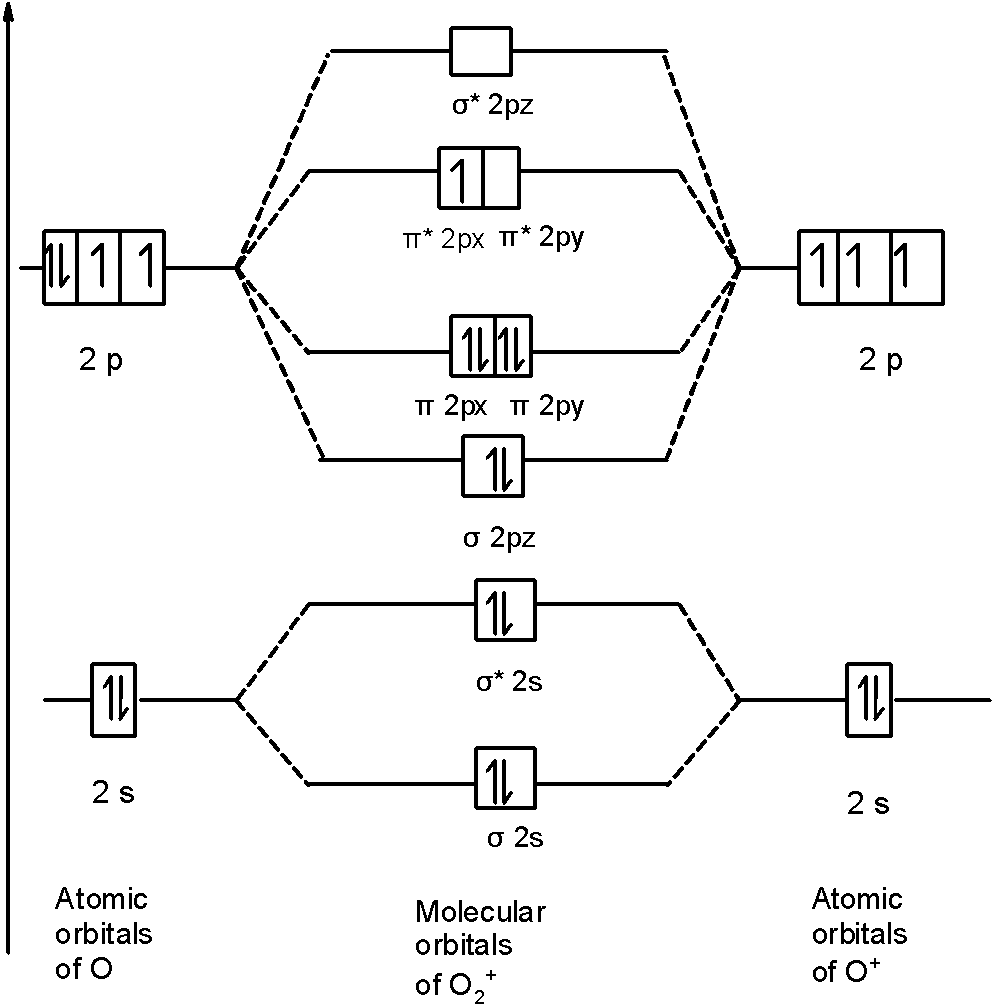
Molecular orbital : A molecule in which all the electrons are paired, is called diamagnetic. | Online Chemistry tutorial IIT, CBSE Chemistry, ICSE Chemistry, engineering and medical chemistry entrance exams, Chemistry Viva, Chemistry Molecular Orbital diagram of O2- ion : This is superoxide ion. In molecular orbital theory, bond order is also defined as the difference, divided by two, between the number of bonding and antibonding electrons; this often, but not always, yields the same result. This MO diagram depicts the molecule H2, with the contributing AOs on the outside sandwiching the MO. Molecular Orbital Diagram Molecules Geometry Molecular Orbital Atomic Orbitals. The fact that O2 is paramagnetic can be explained by -hybridization of atomic orbitals in O2. -the Lewis structure of O2. -the molecular-orbital diagram for O2. -resonance. -a violation of the octet rule. 3) If Nb = Na ,the molecule is again unstable because influence of electrons in the antibonding molecular orbital is greater than the bond influence of electron in the bonding Greater value of bond order for H2 molecule than H2+ ion shows that two H2 molecule is more stable than H2+.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.Chemical bonding of H2O...
Hybridized Molecular Orbital (MO) diagram of H 2O. To further distinguish the electron energy differences between the two non-bonding orbitals, orbital mixing can be further performed between the 2p (3a1) orbital on oxygen and the antibonding 4a1 orbital since they are of the same symmetry and...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
Molecular orbital diagram corresponding to an octahedral MX N complex.... | Download Scientific Diagram
Molecular Orbital Theory. I'm having a lot of trouble with this stuff. I don't really know how to start these questions (such as how to draw a correlation diagram) Feel free to ask clarification questions. I'll use your example of O2-. An orbital correlation attempts to show how the atomic orbitals belonging to...
the molecular orbital-dependent kinetics of COS reactivity, with regard to the experiment by Heinen & Lauwers (1996) and with regard to Schoonen et al.'s Luther (2004) writes the Lewis structure for COS as either OaCaS or ObC-S. At the vent-ocean interface, H 2 S would be interfacing with marine CO 2...
These diatomic molecules should have similar bond orders to the analogous diatomics from the row directly above them in the periodic table O2 has two unpaired electrons in its π* orbitals, and a bond order of 2. The simple Lewis structure has all electrons paired, which does not match the...
The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. A dihydrogen molecule (H2) forms from two hydrogen atoms. When the atomic orbitals of the two atoms combine, the electrons occupy the molecular orbital of lowest energy, the...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.Chemical bonding of H2O...
A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row
File:H2O-MO-Diagram.svg. From Wikimedia Commons, the free media repository. Jump to navigation Jump to search. Added orbital diagrams for molecular orbitals.
Molecular orbital calculations indicate, however, that for O2, F2, and hypothetical Ne2 molecules, the 2p orbital is lower in energy than the 2p orbitals The solid lines represent the relative energies of the indicated atomic and molecular orbitals. (a) The diagram for H2, He2, Li2, Be2, B2, C2, and N2...
- MO diagrams for Inorganic complexes. • It is a waste of both the lecturers and students time if the tutorial to ends up being a lecture covering questions. 5. An introduction to Molecular Orbital Theory.
1- Draw the molecular orbital diagram of transition metal ion in high-spin Mn(H2O)4(OH)2 complex, also determine... - HomeworkLib
Lupine Publishers | Structures and Electrical Properties of Some Biologically Active Nucleic Acid Constituents



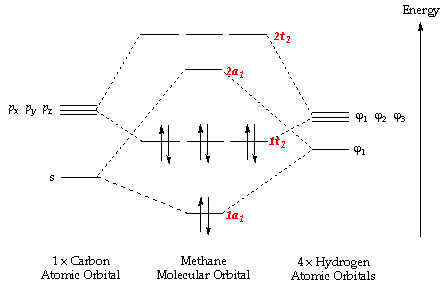










0 Response to "41 molecular orbital diagram for h2o"
Post a Comment