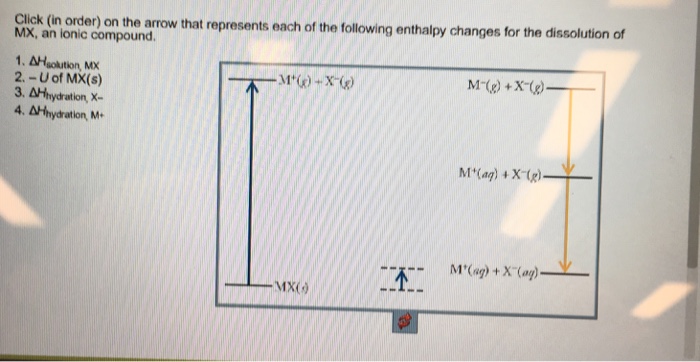
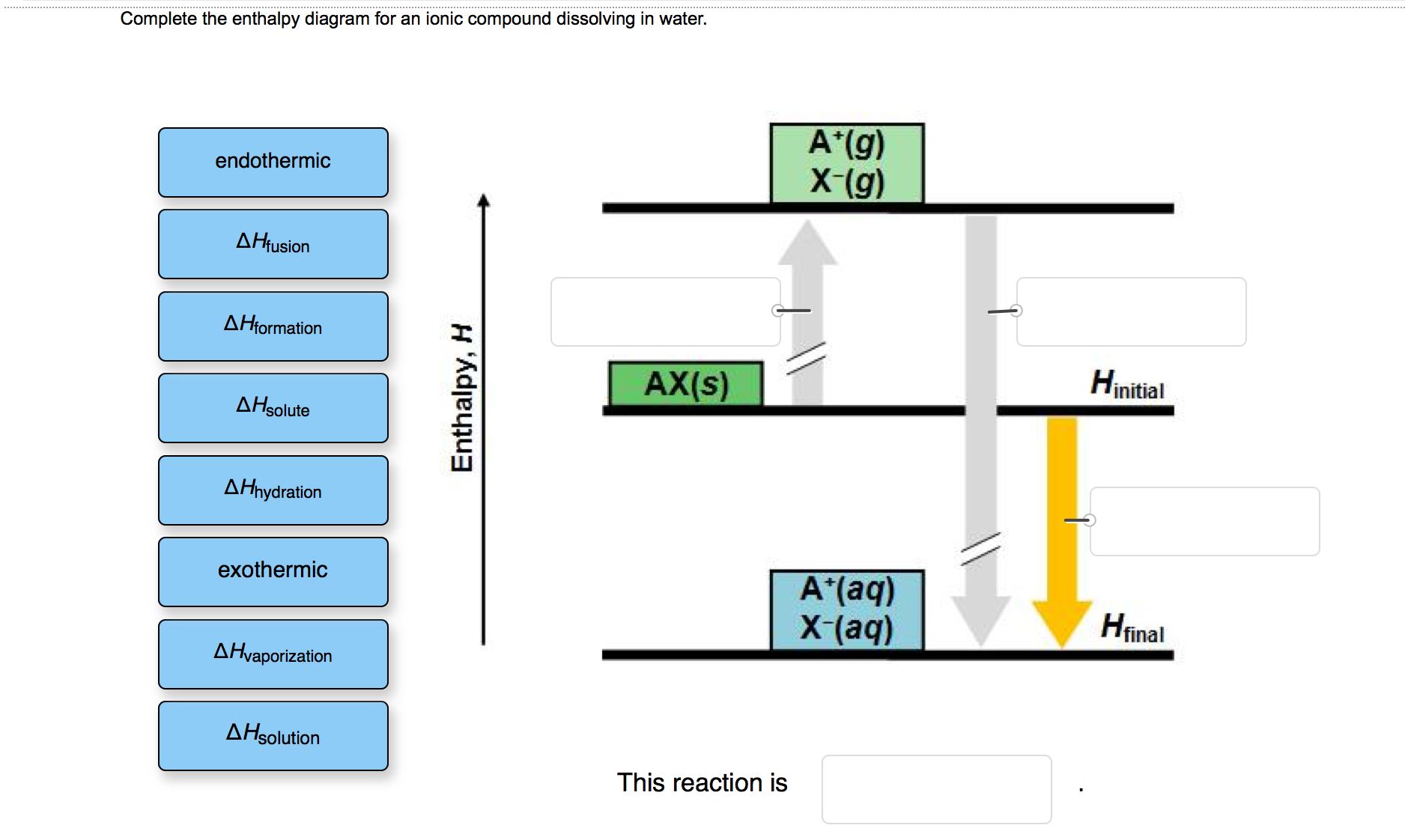
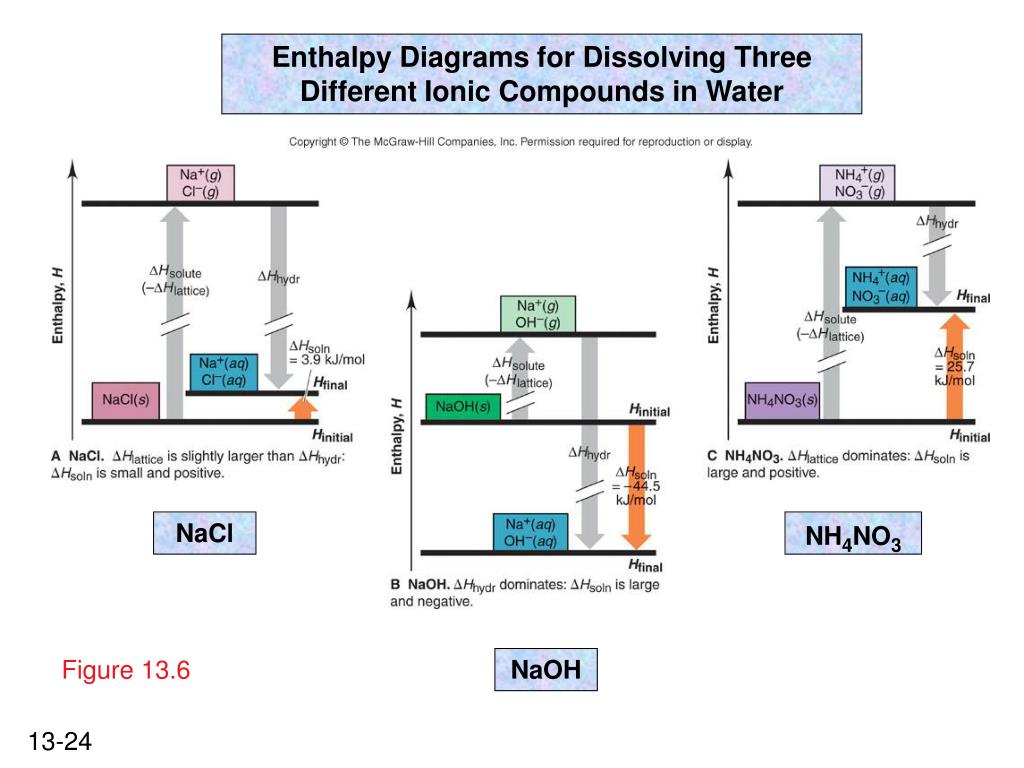
41 complete the enthalpy diagram for an ionic compound dissolving in water.
the enthalpy change when one mole of an ionic compound dissolves in sufficient water to produce a solution of infinite dilution. Enthalpy changes can be determined in the laboratory using ... Get 24⁄7 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply.
A complete enthalpy diagram will include ... An example of this is when we dissolve ammonium chloride in water. If you do this in a test tube you can actually feel the test tube getting colder ...
Complete the enthalpy diagram for an ionic compound dissolving in water.
Complete the enthalpy diagram for an ionic compound dissolving in water b g y g ahsolution b. This enthalpy of solution δhsolution can either be positive endothermic or negative exothermic. With the aid of a diagram show the enthalpy changes involved in the dissolving of an ionic compound in water where enthalpy change of solution is exothermic. Enthalpy change of solution and Enthalpy change of Hydration When ionic compounds dissolve in water, there is usually a temperature change. Sometimes this is exothermic (e.g. dissolving calcium chloride) and sometimes endothermic (e.g. dissolving ammonium nitrate). The experiments are easy to carry out in a laboratory, and the usual equations ... It does not dissolve in water. C. It contains ionic bonds. D. It contains covalent bonds. ... An alkali metal will readily lose an electron and a halogen will readily gain an electron to form an ionic compound with a 1:1 ratio of X+:Z− ions. ... The diagram above is a molecular model of a …
Complete the enthalpy diagram for an ionic compound dissolving in water.. Ionic compounds are often soluble in water, because the attractions formed between ions and water are frequently strong enough to make their solution either exothermic or only slightly endothermic. For example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat endothermic. When ionic compounds dissolve in water, their ions separate from one another in a process called dissociation. One interesting feature of water and many other covalent compounds is that they too can dissociate into ions. Unlike ionic compounds, such as sodium chloride, they are not ionized before they dissociate; they accomplish ionization and ... Complete the enthalpy diagram for an ionic compound dissolving in water b g y g ahsolution b. Using the dissolution of ionic compounds in water to explore thermodynamics. With the aid of a diagram show the enthalpy changes involved in the dissolving of an ionic compound in water where enthalpy change of solution is endothermic. Chemistry. Chemistry questions and answers. Complete the enthalpy diagram for an ionic compound dissolving in water. A (g) X- (g) AHnydration AHusion exothermic AX (s) initial ??,aporization endothermic A (aq) x- (aq) A Hsolution Hrinal ??,ormation AHsolute This reaction is.
Some ionic compounds dissolve readily in water, while others are insoluble. Some ionic com-pounds give o heat when dissolving in water and others absorb heat. Whether the dissolution process of a given ionic compound gives o or absorbs heat depends on the strength of the inter-molecular forces holding the solid together, as well as those ... The Dissolving Process. Water typically dissolves many ionic compounds and polar molecules. Nonpolar molecules such as those found in grease or oil do not dissolve in water. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. Water molecules move about continuously due to ... Many ionic compounds are soluble in water, however, not all ionic compounds are soluble. Ionic compounds that are soluble in water exist in their ionic state within the solution. You will notice in Figure 7.2 that the sodium chloride breaks apart into the sodium ion and the chloride ion as it dissolves and interacts with the water molecules. Terms in this set (70) Combustion Reactions. A reaction in which a substance reacts with oxygen, emitting heat and forming one or more oxygen-containing compounds. Evidence of a chemical reaction: -color change. -formation of a solid in a previously clear solution. -the formation of a gas when we add a substance to a solution.
Calculate the amount of heat required (in kilojoules) to heat 5.00 grams of water from -16.0 C to 11.0 c.-enthalpy of vaporization for water is 40.56 kJ/mol-enthalpy for fusion of water is 6.007 kJ/mol -specific heat for ice is 2.090 J/(gram x *C)-specific heat for water is 4.184 J/(gram x *C)-specific heat for steam is 2.030 J/(gram x *C) Some ionic compounds give off heat when dissolving in water and others absorb heat. Whether the dissolution process of a given ionic compound gives off or absorbs heat depends on the strength of the intermolecular forces holding the solid together, as well as those between the ions and the water once dissolved. (ii) The temperature change in this experiment shows that dissolving lithium iodide in water to form lithium iodide solution is an exothermic process. Complete the energy level diagram to show the position of the lithium iodide solution. Label the diagram to show ûH, the molar enthalpy change. (2) (Total for Question 5 = 11 marks) The relatively strong intermolecular forces resulting from dipole-dipole and H-bond interactions account for properties such as its high boiling point for a small molecule, a high enthalpy of vaporization, large heat capacity, and an ability to dissolve polar and many ionic compounds. In fact, water is a paragon in the realm of hydrogen-bonding ...
Sodium chloride (NaCl) dissolves when water molecules continuously attack the NaCl crystal, pulling away the individual sodium (Na +) and chloride (Cl -) ions. This nonstop attack continuous until the whole NaCl crystal disintegrates. To understand this process at the molecular level, we must apply the three steps we previously discussed.
Ionic compounds dissolve in water because the water molecules hydrate the ions. > To dissolve an ionic compound, the water molecules must be able to stabilize the ions that result from breaking the ionic bond. They do this by hydrating the ions. Water is a polar molecule. It has a permanent dipole. The "O" atom has a partial negative charge, and the "H" atoms have a partial positive charge.
Dissociation. An ionic crystal lattice breaks apart when it is dissolved in water. Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. It is important to be able to write dissociation equations. Simply undo the crisscross method that you learned when writing chemical formulas of ionic compounds.

Please Help Me Find All The True Select All Statements That Are True Concerning The Enthalpy Homeworklib
Dissolution of an ionic compound in water Sodium chloride is a common ionic compound which exists as a solid at room temperature. As a solid, sodium ions (Na+) and chlorine ions (Cl-) are held in a 3D ionic lattice, held together by strong electrostatic forces …
Every compound dissolved in solution broken into ions charges everywhere. Complete the enthalpy diagram for an ionic compound dissolving in water b g y g ahsolution b. The size of the ions involved the charges on the ions. This polarity attracts and pulls apart cations and anions of an ionic compound. Acs gen chem 1 final.
4 *P44255A0420* 2 The solubility of a solid in water is the maximum mass of the solid that can dissolve in 100 g of water at a given temperature. An aqueous solution containing this maximum mass can be described as a saturated solution. The graph shows the solubilities of three solids at different temperatures.
Dissolving ionic compounds in water When an ionic compound dissolves in water two processes occur 1. Energy has to be taken in to break up the lattice and separate the positive and negative ions. This is the lattice enthalpy 2. The ions become surrounded by solvent and bonds form - energy is released when these ions form bonds with water molecules.
Devkit is a vibrant, end-to-end website template pack for open source software projects, SaaS and API services.
complete the enthalpy diagram for an ionic compound dissolving in water. Answer + 20. Watch. 1. answer. 0.
When water dissolves a substance, the water molecules attract and "bond" to the particles (molecules or ions) of the substance causing the particles to separate from each other. The "bond" that a water molecule makes is not a covalent or ionic bond. It is a strong attraction caused by water's polarity.

Please Help Me Find All The True Select All Statements That Are True Concerning The Enthalpy Homeworklib
Chapter 4 Stoichiometry of Chemical Reactions Figure 4.1 Many modern rocket fuels are solid mixtures of substances combined in carefully measured amounts and ignited to yield a thrust-generating chemical reaction. (credit: modification of work by NASA)
Chemistry questions and answers. Complete the enthalpy diagram for an ionic compound dissolving in water B (g) Y (g) AHsolution B' (aq) Y- (aq) Ahydration Ht final exothermic H solute AHvaporization BY (S) Hinitial endothermic Hormation This reaction is.
It is an oxygen hydride, a mononuclear parent hydride and an inorganic hydroxy compound. It is a conjugate base of an oxonium. It is a conjugate acid of a hydroxide. Water is h2O, a clear, colorless, odorless, tasteless liquid that freezes into ice below 0 degrees centigrade and boils above 100 degrees centigrade.
a. Water, H 2O ionic compound covalent compound b. Sodium chloride, NaCl ionic compound covalent compound c. Calcium carbonate, CaCO 3 ionic compound covalent compound d. Hydrogen chloride, HCl ionic compound covalent compound e. Glycerol, C 3H 8O 3 ionic compound covalent compound 3. Nitrate is a polyatomic ion with a charge of -1.
It does not dissolve in water. C. It contains ionic bonds. D. It contains covalent bonds. ... An alkali metal will readily lose an electron and a halogen will readily gain an electron to form an ionic compound with a 1:1 ratio of X+:Z− ions. ... The diagram above is a molecular model of a …
Enthalpy change of solution and Enthalpy change of Hydration When ionic compounds dissolve in water, there is usually a temperature change. Sometimes this is exothermic (e.g. dissolving calcium chloride) and sometimes endothermic (e.g. dissolving ammonium nitrate). The experiments are easy to carry out in a laboratory, and the usual equations ...
Complete the enthalpy diagram for an ionic compound dissolving in water b g y g ahsolution b. This enthalpy of solution δhsolution can either be positive endothermic or negative exothermic. With the aid of a diagram show the enthalpy changes involved in the dissolving of an ionic compound in water where enthalpy change of solution is exothermic.
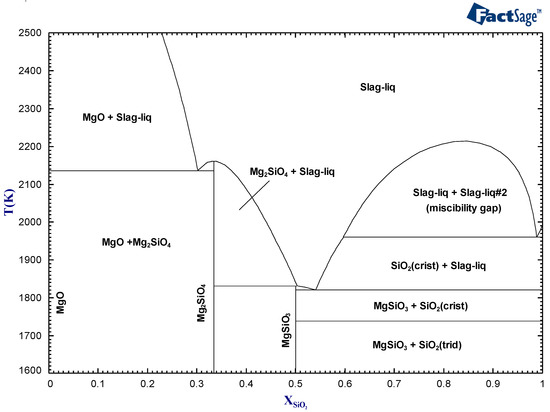
Processes Free Full Text On The Application Of The Factsage Thermochemical Software And Databases In Materials Science And Pyrometallurgy Html

Production And Applications Of Amylose Lipid Complexes As Resistant Starch Recent Approaches Li 2021 Starch St 228 Rke Wiley Online Library

Functional Material Systems Based On Soft Cages Liu 2021 Chemistry 8211 An Asian Journal Wiley Online Library
Solved Sketch An Enthalpy Diagram For The Process Of Dissolving Mathrm Nai S In Mathrm H 2 Mathrm O Exothermic

When An Ionic Solid Dissolves In Water Two Processes Occur Firstly The Ions Are Separated Endothermic Secondly The Ions Are Surrounded By Water Ppt Download

A Level What Happens When An Ionic Compound Dissolves In Water Energetics Lattice Enthalpy Enthalpy Of Hydration Solution Ks5 Gce Chemistry Revision Notes

When An Ionic Solid Dissolves In Water Two Processes Occur Firstly The Ions Are Separated Endothermic Secondly The Ions Are Surrounded By Water Ppt Download

Review On Metal Dissolution Characteristics And Harmful Metals Recovery From Electronic Wastes By Supercritical Water Sciencedirect







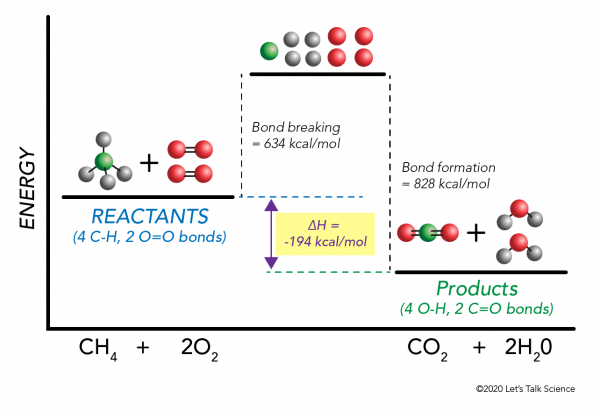
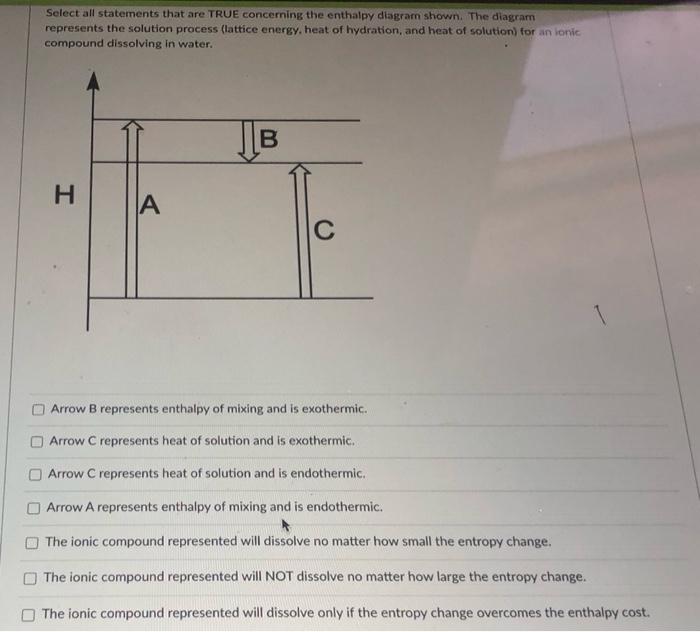

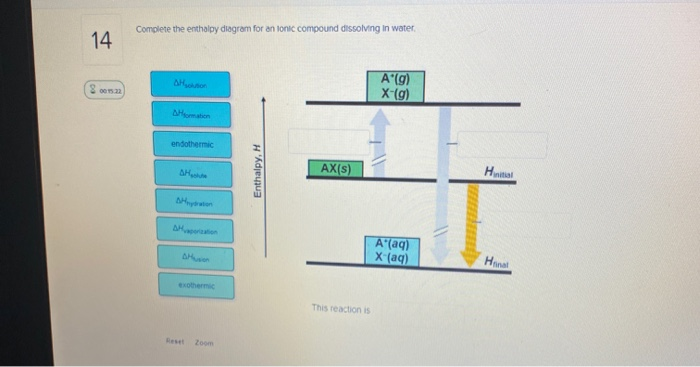


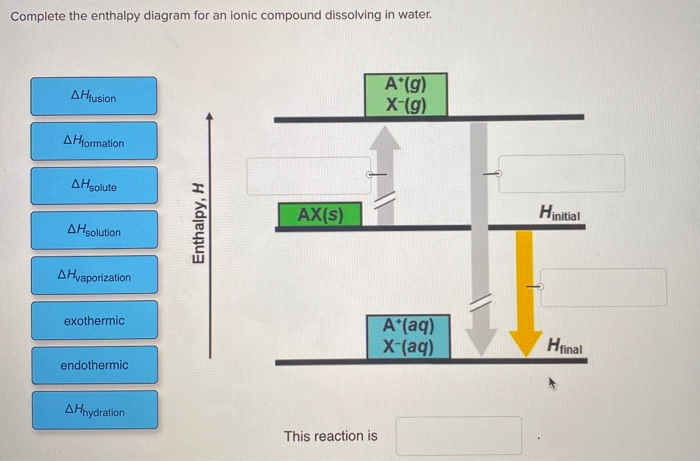


0 Response to "41 complete the enthalpy diagram for an ionic compound dissolving in water."
Post a Comment