40 copper electron dot diagram
What is the Lewis dot diagram for copper? Organic Chemistry Lewis Structures and Bonding Lewis Dot Diagram. 1 Answer. Karen L. Aug 2, 2016. Chemical symbol: Cu. Number of electrons: 29. 1 on the valence shell. Answer link. A step-by-step explanation of how to draw the CuSO4 Lewis Dot Structure.For CuSO4 we have an ionic compound and we need to take that into account when we dra...
Sulfur wants to accept 2 electrons for a full valence shell (see it's column in the periodic table). · So, copper donates 2 electrons to sulfur to form an ionic ...1 answer · 3 votes: Copper wants to donate 2 electrons for a full valence shell (see it’s column in the ...

Copper electron dot diagram
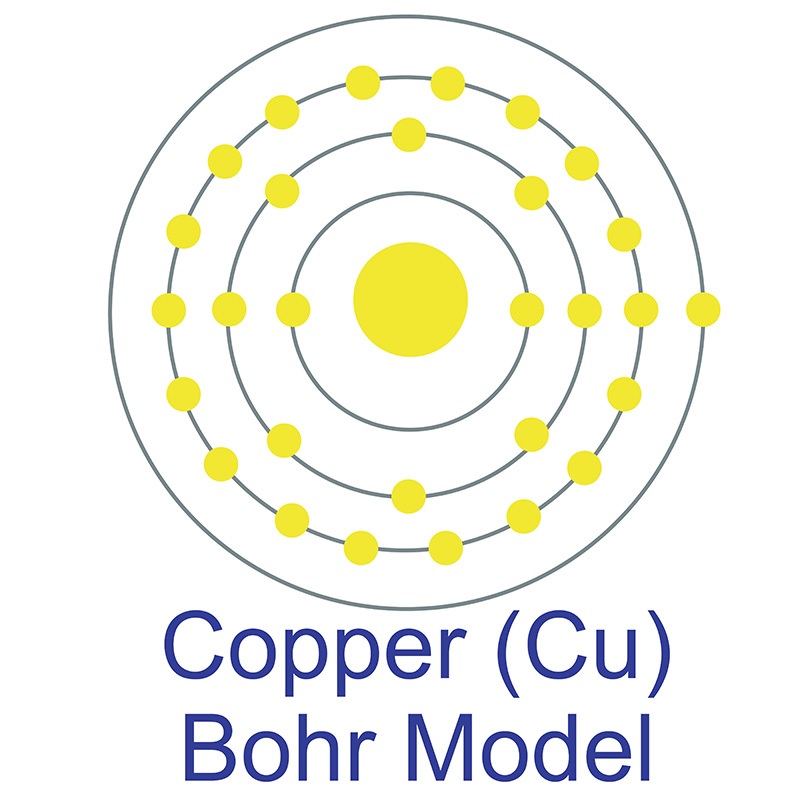
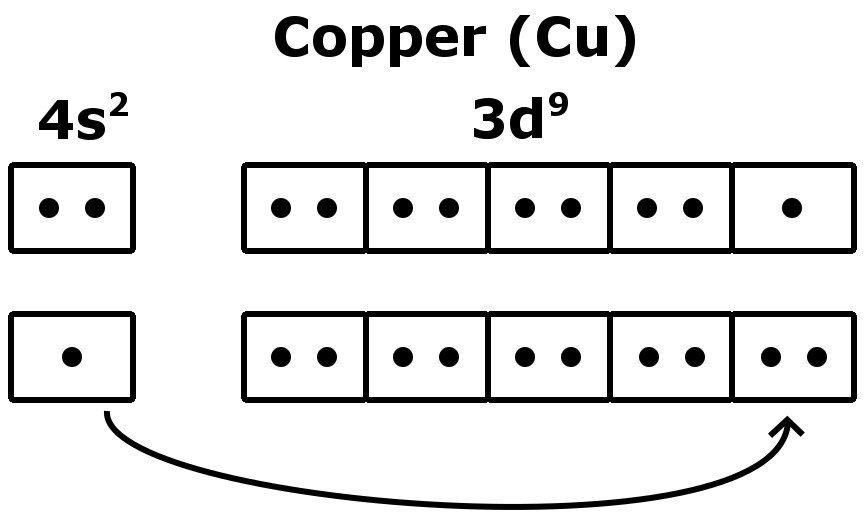
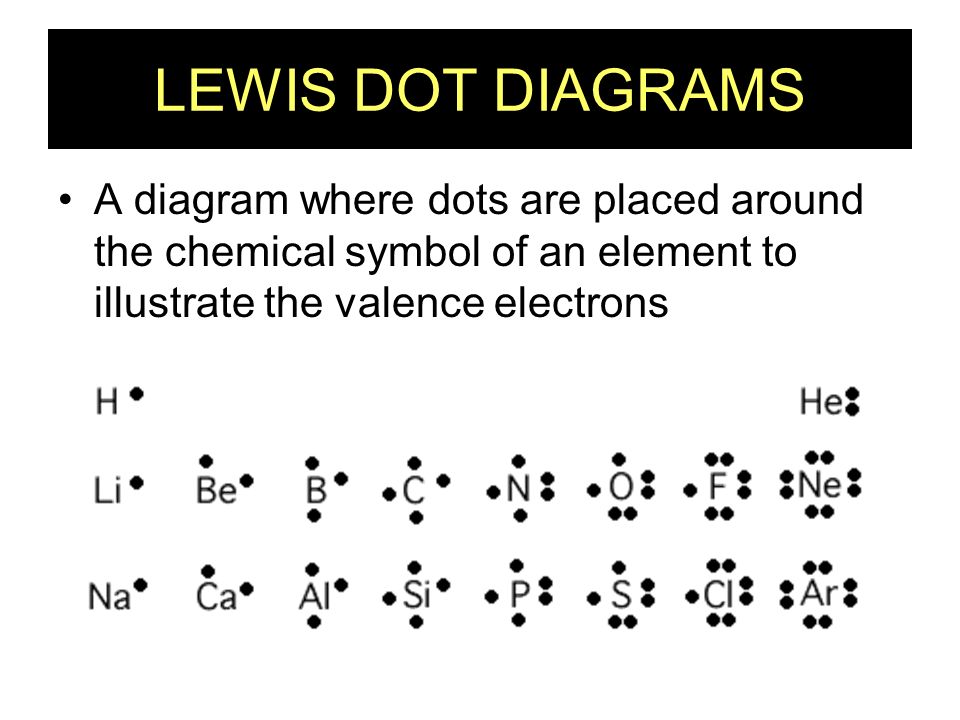
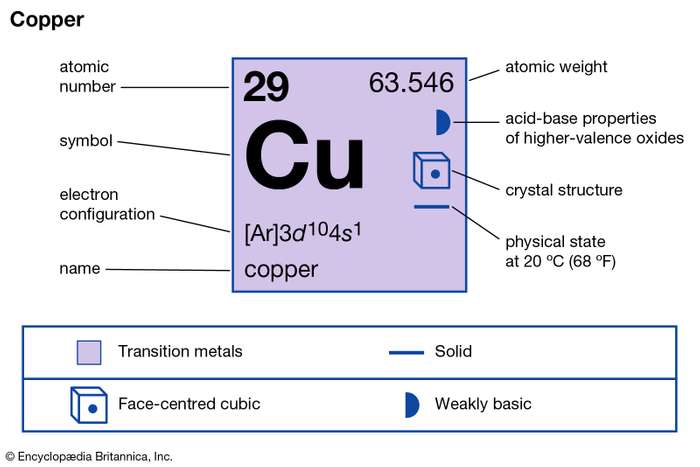
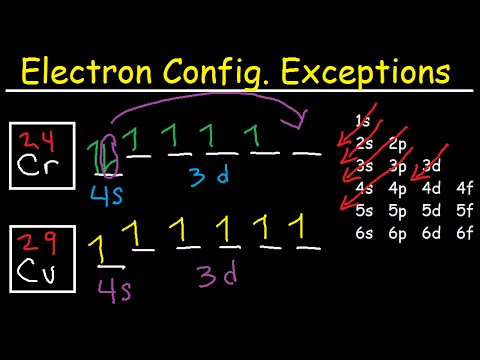
Correct Electron Configuration for Copper (Cu) Half-filled and fully filled subshell have got extra stability. Therefore, one of the 4s2 electrons jumps to the 3d9. This give us the (correct) configuration of: 1s2 2s2 2p6 3s2 3p6 3d10 4s1. For the Cu+ ion we remove one electron from 4s1 leaving us with: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 The lewis structure for an element or ion can be drawn by representing the electrons of the valence shell as dots. The octet of the atom should be kept in mind ...1 answer · Top answer: The atomic number of Cu=29 The electronic configuration of its valence shell =3d10,4s13d10,4s1 Its lewis dot structure can be drawn as... Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element’s symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
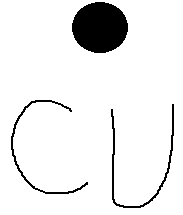
Copper electron dot diagram. Atomic Structure of Copper · Atomic Radius: 1.57Å · Atomic Volume: 7.1cm3/mol · Covalent Radius: 1.17Å · Cross Section (Thermal Neutron Capture)σa/barns: 3.78 ... A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ... Electron-dot diagrams can help you predict how atoms might bond. To draw an electron-dot diagram, write the symbol of the element and place one dot around the symbol for every valence electron in the atom, as shown in Figure 3. Place the first four dots alone on each side, and then pair up any remaining dots. Figure 3 Using Electron–Dot Diagrams Best Answer. Copy. The Lewis dot diagram (structure) for copper would be Cu with a dot on top, because if you look at the shell model of copper, there is only one dot on the outer ring. So the ...
Cu and it needs to have 11 dots around the Cu Copper is an exception to electron configurations. It is has a partially filled 4s orbital and a full d orbitial [Ar] 4s13d10 instead of [Ar] 4s23d9 ... to complete a Lewis electron-dot diagram, including any lone (non-bonding) pairs of electrons when given an array of atoms arranged to represent ethanol. Part (a)(ii) assessed knowledge of structural isomers by asking students to draw a complete Lewis electron-dot diagram for the isomer of the compound drawn in part (a)(i). What is the electron dot diagram for copper? What is An Electron Dot Diagram? Connect with: Register or Login. Questions; What is the electron dot diagram for copper? AskBug. A clean and minimal question and answer theme for WordPress and AnsPress. Theme can be used to create a professional Q&A community. Social. Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element’s symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
The lewis structure for an element or ion can be drawn by representing the electrons of the valence shell as dots. The octet of the atom should be kept in mind ...1 answer · Top answer: The atomic number of Cu=29 The electronic configuration of its valence shell =3d10,4s13d10,4s1 Its lewis dot structure can be drawn as... Correct Electron Configuration for Copper (Cu) Half-filled and fully filled subshell have got extra stability. Therefore, one of the 4s2 electrons jumps to the 3d9. This give us the (correct) configuration of: 1s2 2s2 2p6 3s2 3p6 3d10 4s1. For the Cu+ ion we remove one electron from 4s1 leaving us with: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10

Do Now Finish This Table From Yesterday Formulachemical Namelewis Dot Picture Nabr Calcium Chloride Li 2 S Aluminum Bromide Sodium Bromide Cacl 2 Albr Ppt Download
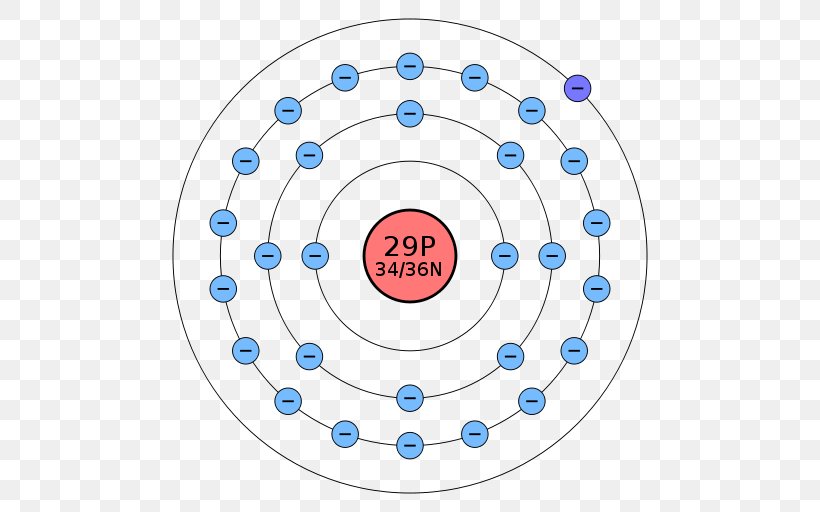
Bohr Model Atom Electron Shell Copper Png 512x512px Bohr Model Area Atom Atomic Number Atomic Orbital
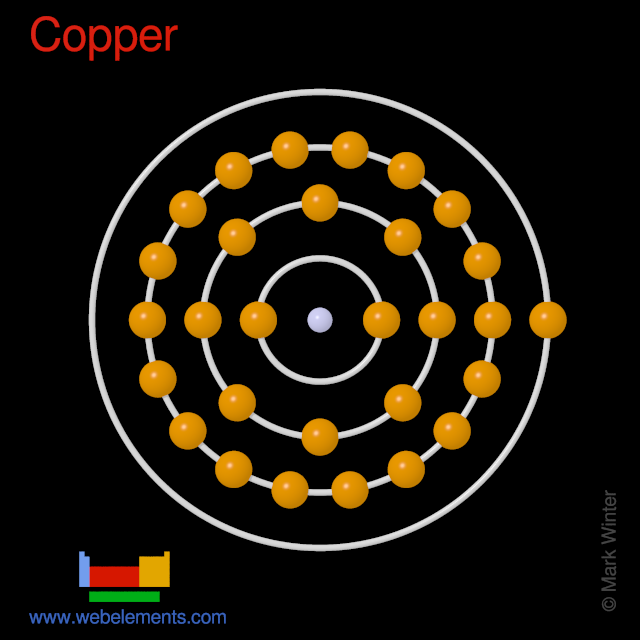
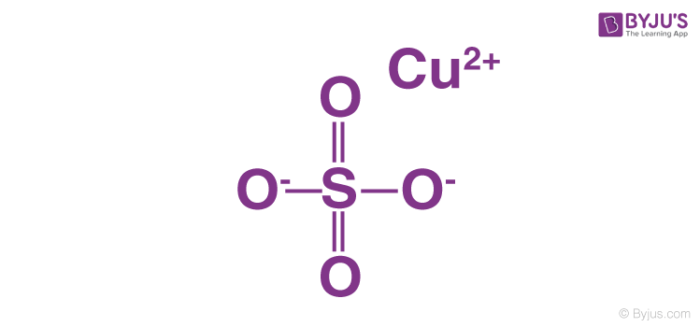
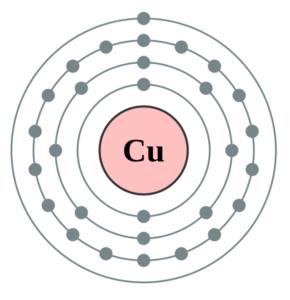

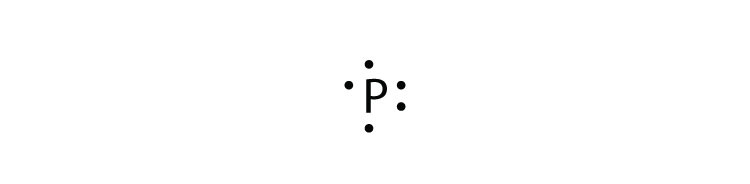




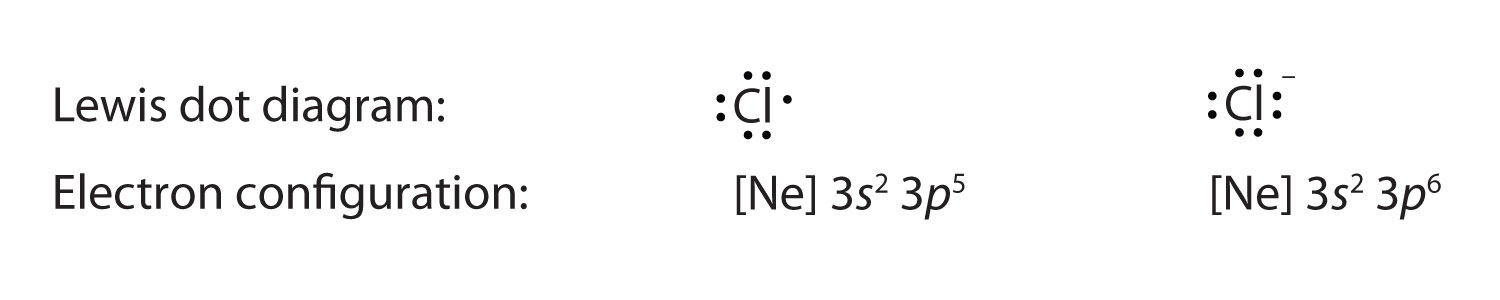










0 Response to "40 copper electron dot diagram"
Post a Comment