39 reaction coordinate diagram endothermic
If a prediction is not possible because the sign of AG will be temperature dependent, describe how AG will be affected by raising the temperature. (a) An endothermic reaction for which the system exhibits an increase in entropy (b) An exothermic reaction for which the system exhibits an increase in entropy. 5. Consider the E diagrams below. Exothermic Reaction Coordinate Diagram Exothermic Process - Assignment Point Exothermic - Key Stage Wiki; What are the conditions for an exothermic vs. an endothermic reaction? | Socratic Exothermic Reactions - Definition and Examples Exothermic and Endothermic chemical changes — lesson. Science State Board, Class 8.
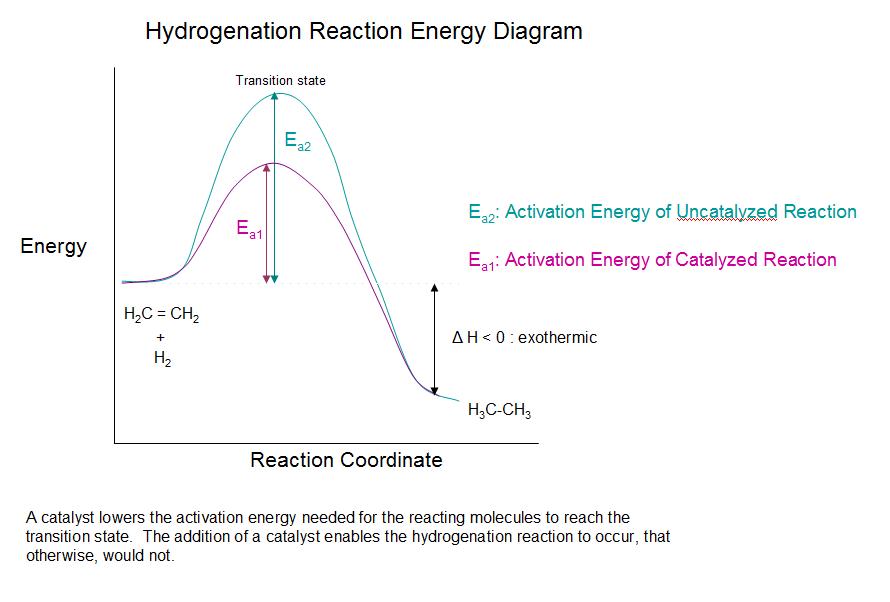
Hydrogenation is the process where hydrogen atoms bind to the double bond of a compound, facilitating its conversion to a single bond, in the presence of a catalyst. Hydrocarbons with double bonds ...
Reaction coordinate diagram endothermic
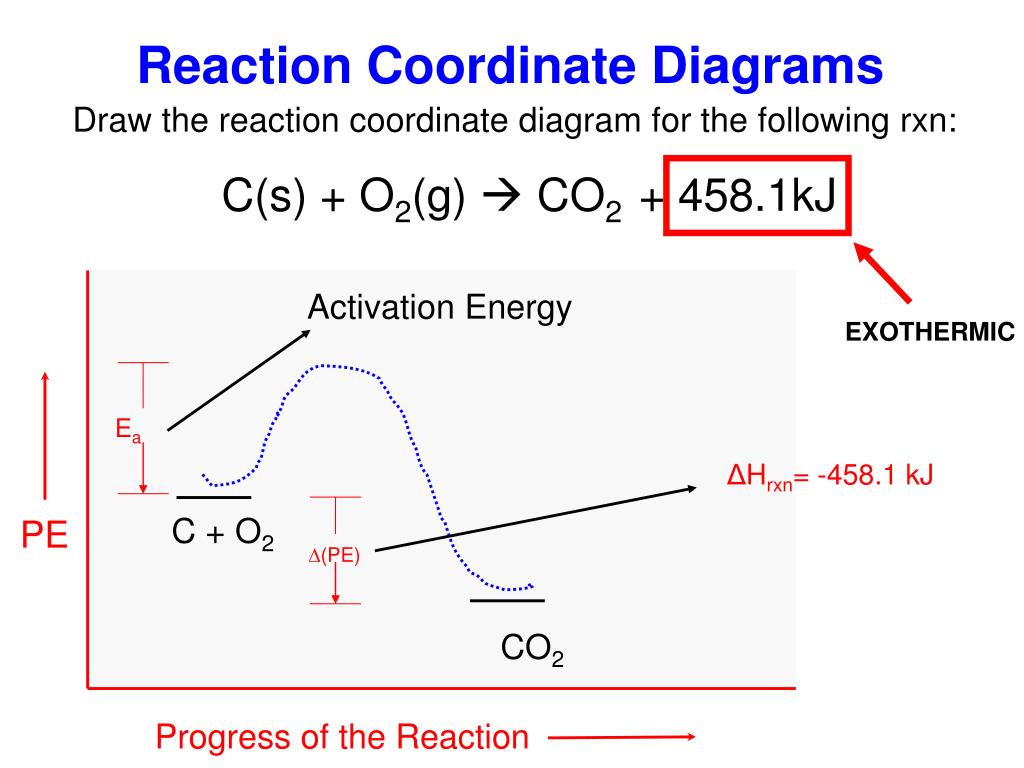
A typical reaction coordinate diagram for a mechanism with a single step is shown below: Below is a reaction coordinate diagram for an endo the rmic reaction. The fully filled in reaction coordinate diagram is displayed below. This reaction is also exo the rmic because the energy of the products is lower than that of the. Since in the first reaction heat is absorbed ,therefore it is endothermic reaction while in the second reaction heat is released,therefore it is exothermic reaction. The reactions are as follows. ... Q27.The diagram shows the reaction between metal and dil.acid. ... Chapter 3- Coordinate Geometry: Chapter 11-Construction: How to draw enthalpy diagram s from a chemical reaction an d a dH value.Table of Contents:00:14 - Learning Objectives A ch an ge in enthalpy is the difference between the enthalpy of the products an d the enthalpy of react an ts. To draw a entity relationship diagram for bus services?
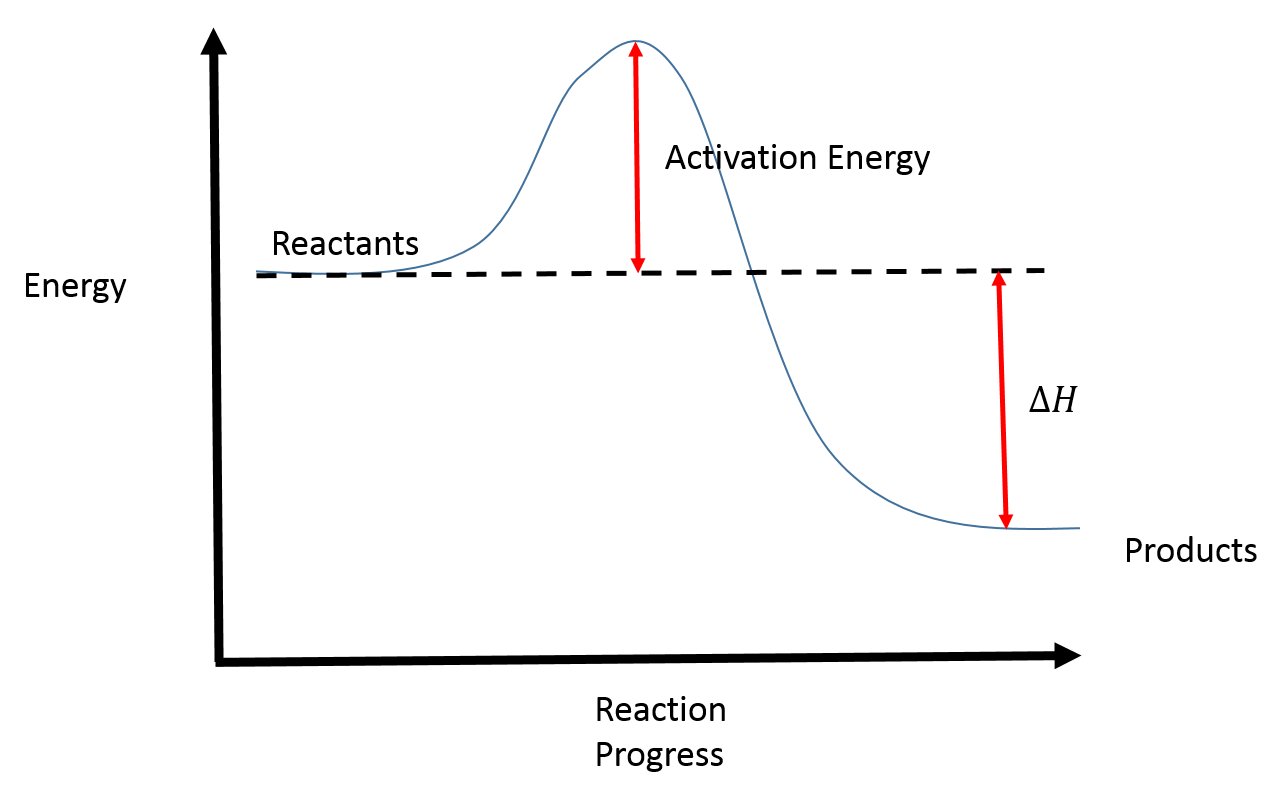
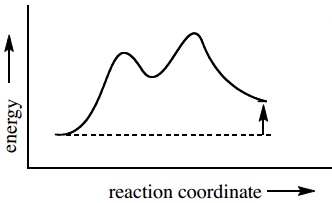
Reaction coordinate diagram endothermic. Exothermic Reactions - The opposite of an endothermic reaction is an exothermic reaction. It releases energy to its surroundings in the form of light or heat. Neutralization, burning a chemical, fuel reactions, deposition of dry ice, respiration, sulfuric acid solution in water, and many more processes are examples. The fully filled in reaction coordinate diagram is displayed below. The arrow marked in the question represents the activation energy, which is the energy barrier that must be overcome in order for the reactants to form products. This reaction is also exothermic because the energy of the products is lower than that of the reactants. A Draw a reaction coordinate diagram that is consistent with ... Answers: 3 on a question: What type of reaction is being shown in this energy diagram? Energy Products Activation Energy Reactants to ti Time A. Endothermic, because energy is released to the surroundings B. Endothermic, because energy is absorbed from the surroundings ОО O C. Exothermic, because energy is absorbed from the surroundings D. Exothermic, because energy is released to the ... A reaction coordinate diagram is a graph that plots energy versus reaction progress. The amount of energy that needs to be added is called the activation energy, which is the point where the line on the graph is curving up. ... Endothermic reactions in organisms are called anabolic reactions. What is a hydrolysis reaction? Thus, a hydrolysis ...
Download File PDF Chapter 8 Covalent Bonding Worksheet Answer Key Chapter 8 Covalent Bonding Worksheet Answer Key When somebody should go to the book stores search establishment by shop shelf by shelf it is essentially problematic. During a actinic reaction. Covalent Bond Webquest Name Sci 1 Directions Answer The Following By Going To The Notes […] This reaction is endothermic. Write out the balanced chemical reaction and draw a reaction coordinate diagram, as well as a PEC diagram. Which direction would the reaction go at low temperature? Two sealed vials of air (N2, O2, and CO2) are heated to allow the particles to react. Two separate Potential Energy Diagrams. Graphs of the energy changes that action during a actinic reaction. % Progress. Diagrams of activation energy and acknowledgment advance are given. Review. In an endothermic reaction, is the abeyant energy of the articles college or lower than the abeyant energy... Some of the worksheet s for this concept are Potential energy diagram work answers, Work 1 2 potential energy diagram s key, Ws 4 potential energy diagram s work, Name kinetics potential energy diagram s, Work 1 2 potential energy diagram s, Chemistry 12 work 1 2, Energy diagram s, Forms of energy lesson plan chemical energy. Unit 1-Reaction ...
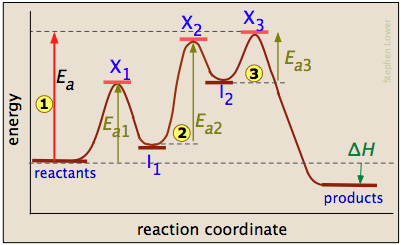
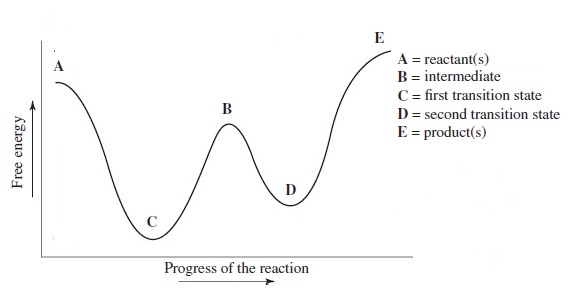
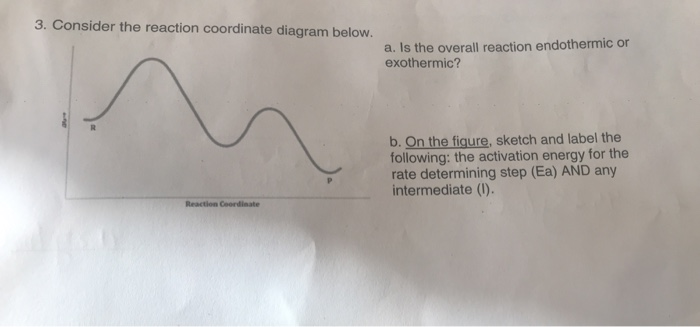
B Reaction Coordinate Which lettered interval on the diagram represents the potential energy of the products? The energy changes that occur during a chemical reaction can be shown in a diagram called a potential energy diagram, or sometimes called a reaction progress curve. Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DG rxn, as well as DG 1 * and DG 2 * for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk. Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra... I am confused on whether in a reaction coordinate diagram, does the difference between the reactants and products equal deltaH or deltaG I have seen graphs that demonstrate both. If some could explain that would be great!   for reference this khan article states it as deltaH: https://www.khanacademy.org/test-prep/mcat/chemical-processes/thermochemistry/a/endothermic-vs-exothermic-reactions   This khan article states it as deltaG: https://www.khanacademy.org/science/biolog...
Reactants reaction coordinate a is the reaction exothermic or endothermic. 2nd law of thermodynamics m. A worksheet that consists of 10 questions that involves solving molar conversions with an answer key that can be used for a pop quiz student assessment check for understanding homework assignment and more. B what is the sign of h.
Exothermic Reaction Coordinate Diagram Exothermic Reaction Illustration - Twinkl Exothermic Vs Exergonic Reactions: Know the difference Exothermic and Endothermic chemical changes — lesson. ... Calculate Energy Changes in Exothermic and Endothermic Reactions Worksheet - EdPlace Exothermic Standard Version - Exothermic ...
Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra...
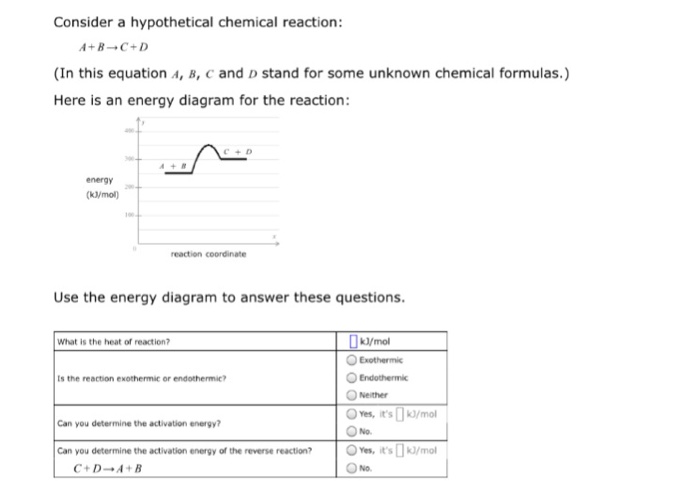
Elements And Their Properties Worksheet Answers Promotiontablecovers. Diagram Potential Energy Instructioal Fair Answers Full. H cell body cyton. POTENTIAL ENERGY DIAGRAM. Label the axis PE of reactants 350 KJmol Ea 100 KJmol PE of products 250 KJmol. Is the overall reaction as shown exothermic or endothermic. Reaction Coordinate - A B - C ...
Answer (1 of 4): Is there a rapid gas-producing reaction that is not exothermic? Can we make a "cold (or neutral) gunpowder"? To "Expand" (pardon the pun) on the answer by Bill Collis… If you take a liquefied gas, such as liquid nitrogen and leave it in a sealed container, it will eventually bu...
Read also methane and understand more manual guide in the reaction of methane and water is one way The reaction of methane and water is one way to prepare hydrogen for use as a fuelCH4g H2Og COg 3 H2gIf you begin with 995 g of CH4 and 2510 g of watera Which reactant is the limiting reactantb What is the maximum mass of H2 that can be preparedc ...
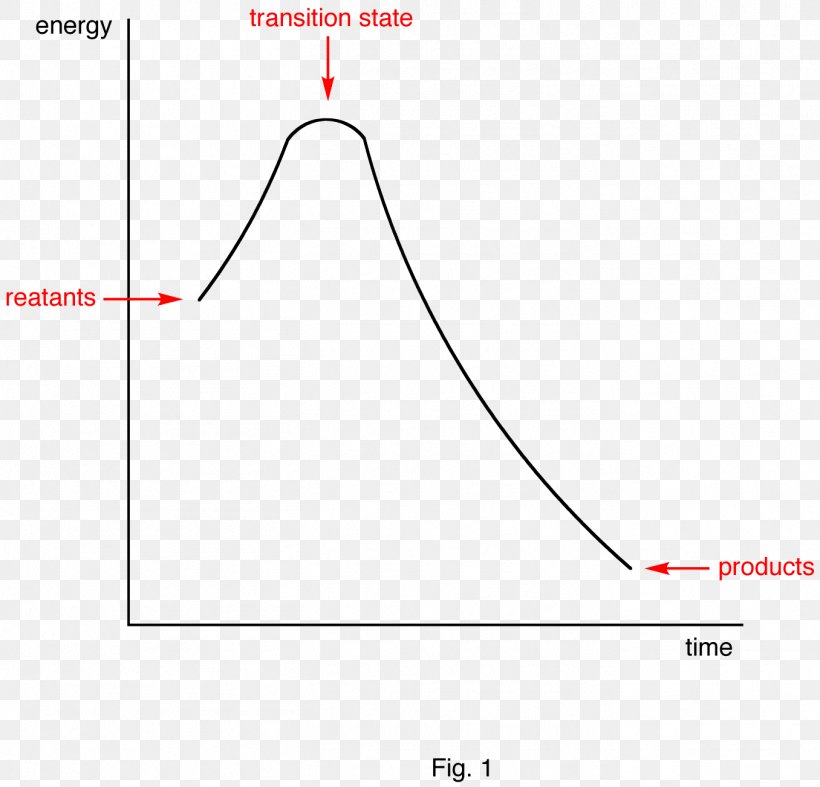
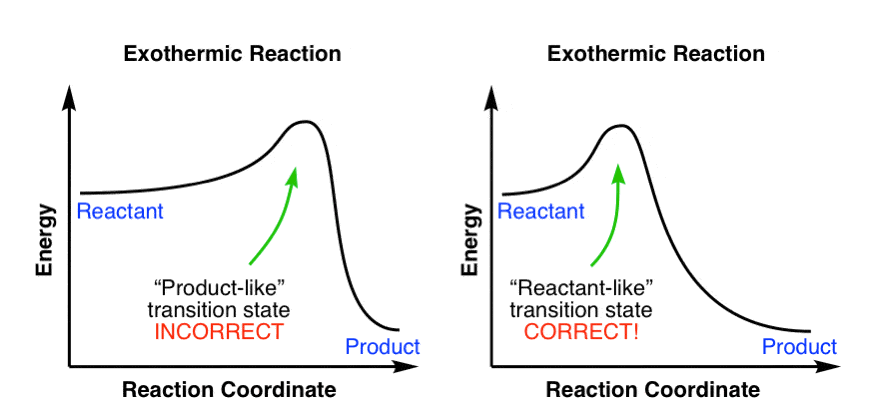
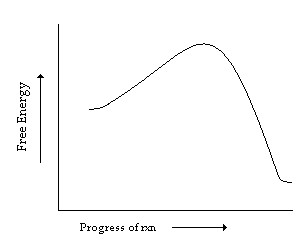
What is an energy diagram? Energy diagrams are used to represent the change in energy for the molecules involved in a chemical reaction. The free energy is measured along the y-axis, and the reaction coordinate is plotted on the x-axis. The reaction coordinate indicates the progress of the conversion of reactants to products.
2 A potential energy diagram for a reaction is shown below. Potential Energy (k/mon Reaction Coordinate State whether the reaction is exothermic or endothermic. b) Indicate on the diagram where the ac. 1 answer Quantitative Analysis Write the Kb reaction of CN- with water. Given that Ka for HCN is 6.2 × 10-10 at 25 °C, what is Kb for CN- ?
Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra...
The allylic and benzylic cations are stabilized by resonance forms as we have seen before. A more detailed ranking including 1°, 2° and 3° allylic and benzylic cations is: 2. Energy Diagrams Energy diagrams map out the energy transitions throughout the steps in a reaction. Reaction Coordinate: Steps of the reaction as it proceeds from reactants to products.
13.10.2021 · Converting intermittent renewable energy resources, such as solar and wind energy, into storable chemical fuels is a key pathway toward a sustainable …This potential energy diagram shows the effect of a catalyst on the activation energy.The catalyst provides a different reaction path with a lower activation energy.As shown, the catalyzed pathway involves a two-step mechanism ...
An endothermic reaction results in products at higher energy than reactants and absorbs energy. Finally, the shorter the bond, the stronger the bond. Finally, the shorter the bond, the stronger ...
Topic: 200 - 150 100 - 50 a b c Reaction Coordinate Does this potential energy diagram represent an exothermic or an endothermic reactio. S Gardencity K12 Ny Us Cms Lib Ny01913305 Centricity Domain 586 4 29 14 20pe 20diagram 20review 20key Pdf Which Numbered Interval Represents The Heat Of Reaction: Content: Summary: File Format: PDF
Endothermic Reaction Coordinate Diagram; What are Endothermic Reactions? (with Examples & Video) Endothermic/ Exothermic; Illustrated Glossary of Organic Chemistry - Endothermic Endothermic Reaction Photograph by Science Photo Library Endothermic Reaction Photograph by Science Photo Library ...
How to draw enthalpy diagram s from a chemical reaction an d a dH value.Table of Contents:00:14 - Learning Objectives A ch an ge in enthalpy is the difference between the enthalpy of the products an d the enthalpy of react an ts. To draw a entity relationship diagram for bus services?
Since in the first reaction heat is absorbed ,therefore it is endothermic reaction while in the second reaction heat is released,therefore it is exothermic reaction. The reactions are as follows. ... Q27.The diagram shows the reaction between metal and dil.acid. ... Chapter 3- Coordinate Geometry: Chapter 11-Construction:
A typical reaction coordinate diagram for a mechanism with a single step is shown below: Below is a reaction coordinate diagram for an endo the rmic reaction. The fully filled in reaction coordinate diagram is displayed below. This reaction is also exo the rmic because the energy of the products is lower than that of the.
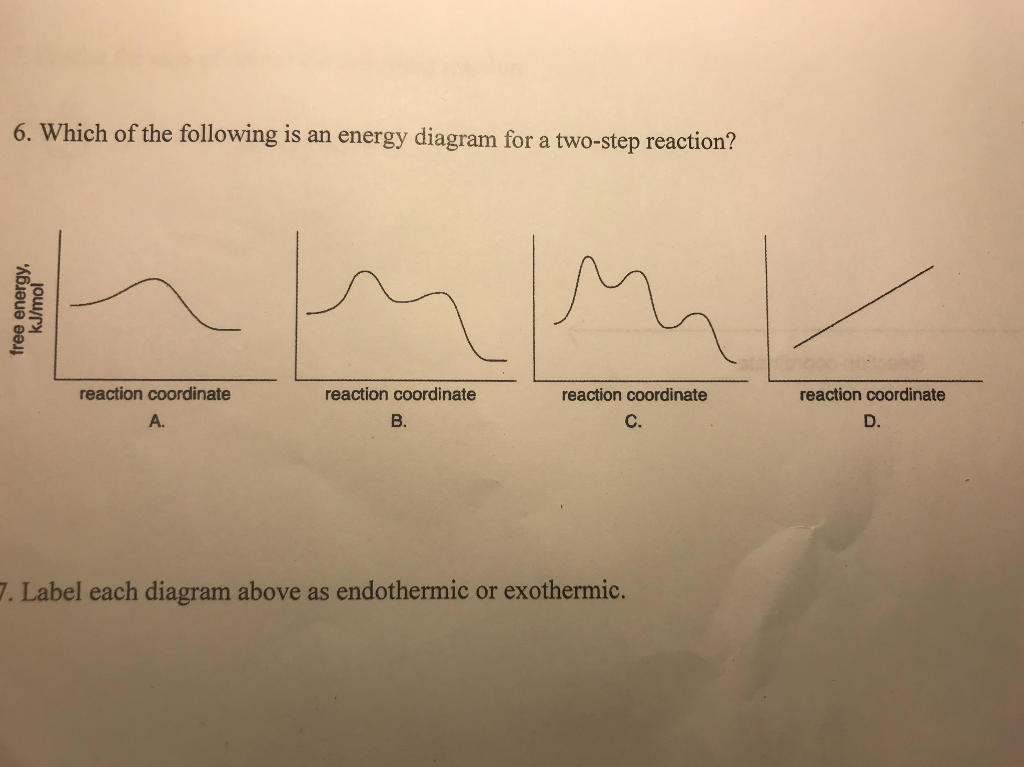

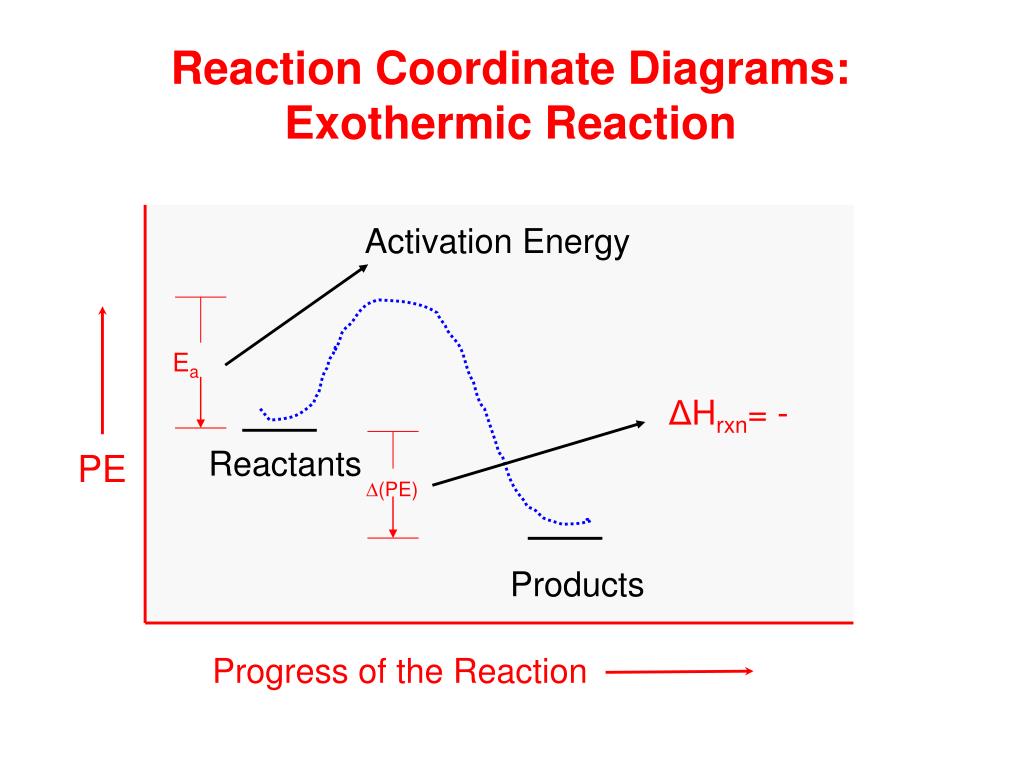
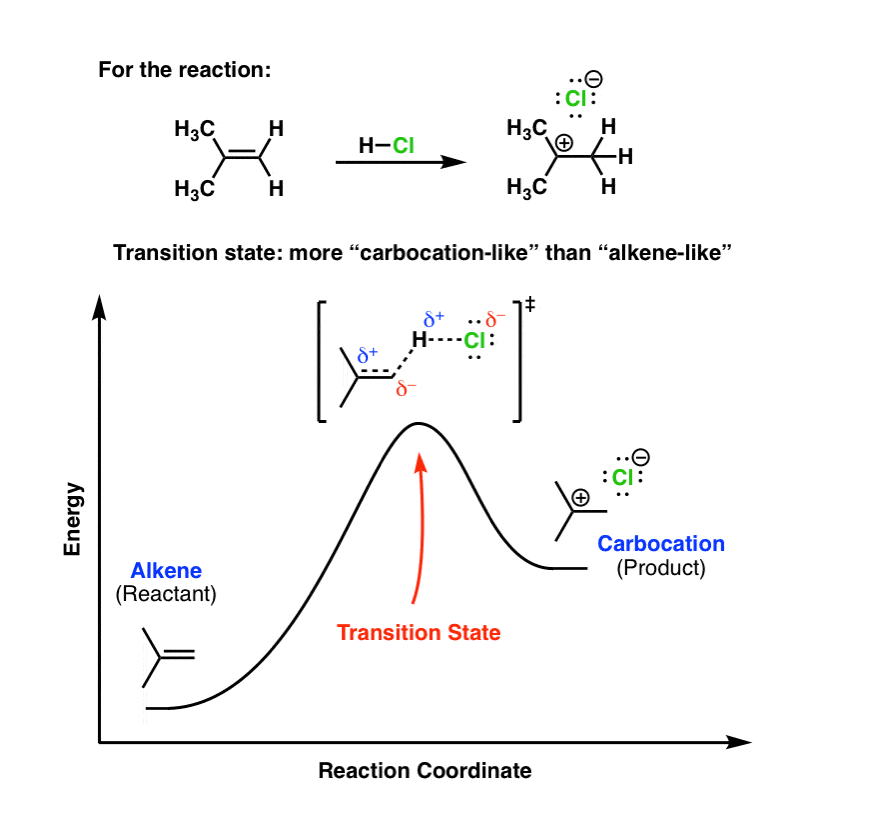

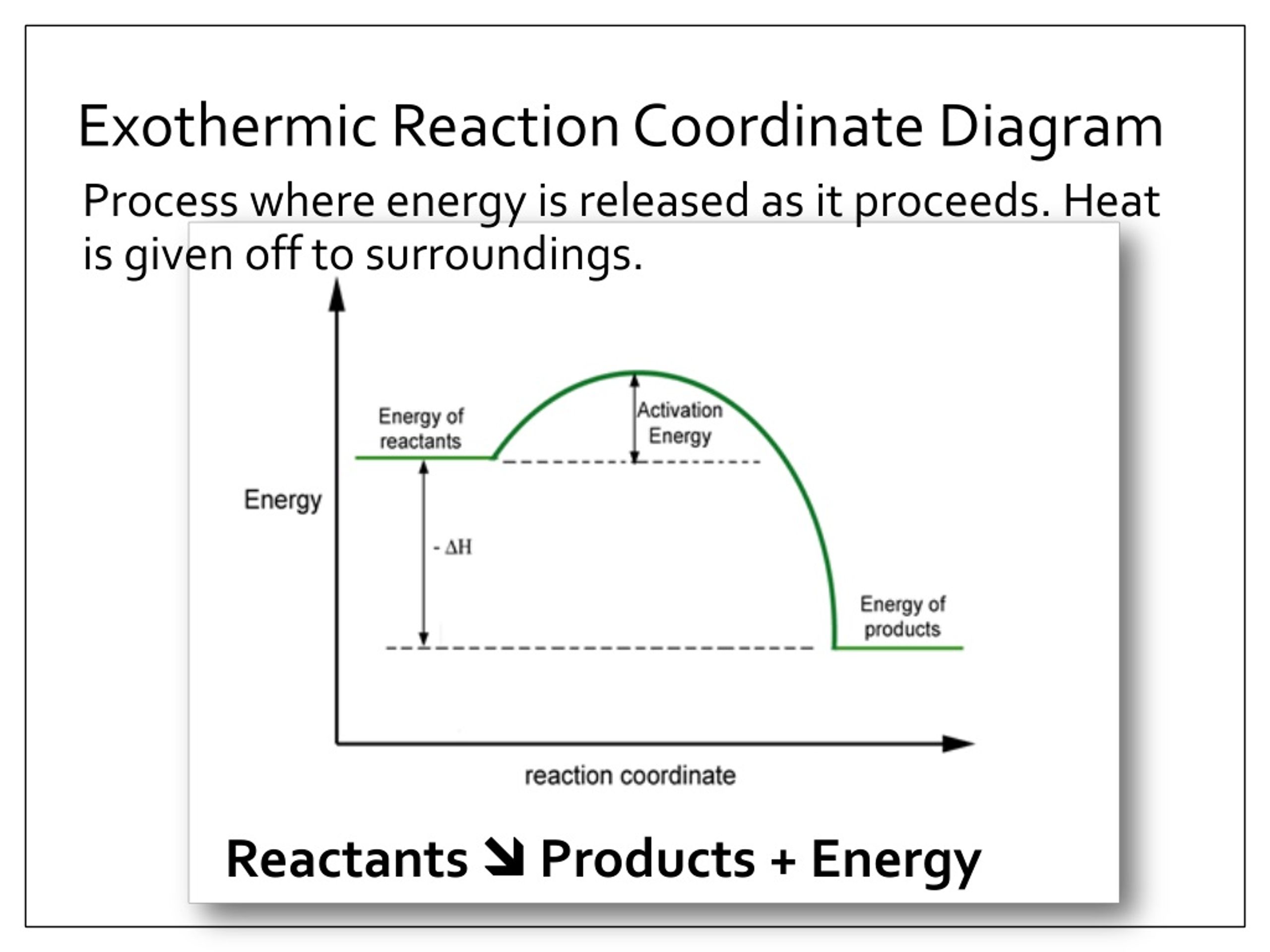











0 Response to "39 reaction coordinate diagram endothermic"
Post a Comment