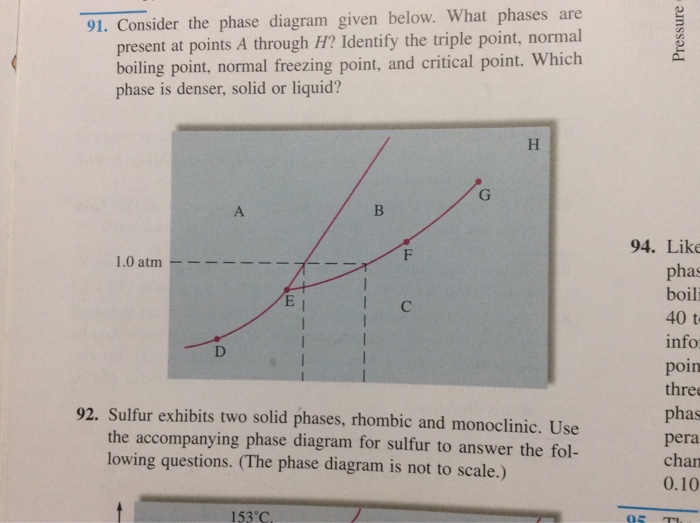
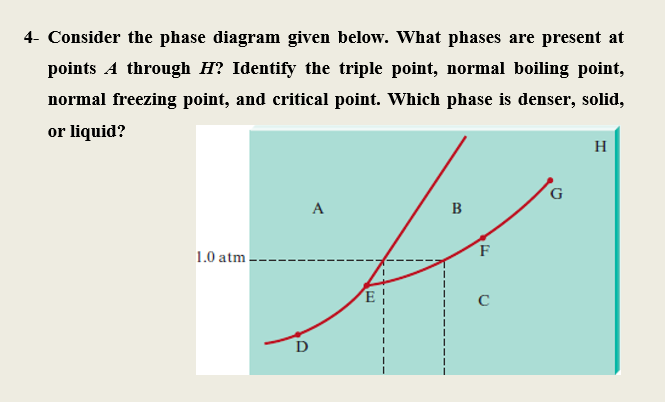
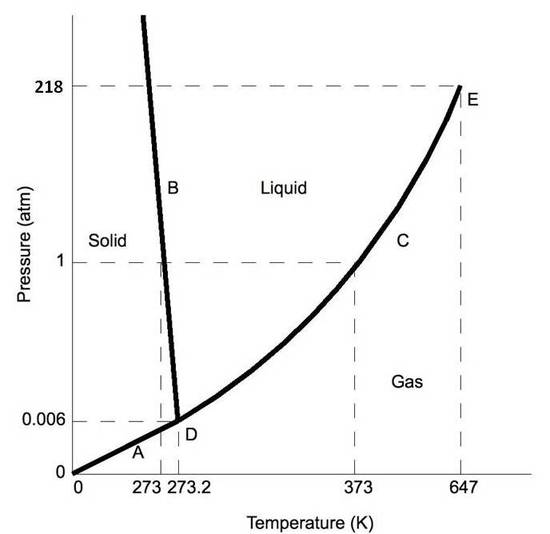
39 examine the following phase diagram and determine what phase(s) exists at point a.
3-Dimensional Depiction of Temperature-Composition Phase Diagram of Bismuth, Tin, and Lead at 1atm. The diagram has been simplified by omission of the regions of solid solubility. Each face of the triangular a eutectic. There is also a peritectic point in the Bi-Pb phase diagram. Figure by MIT OCW. as axes are called phase diagrams. DEF. A phase diagram (or equilibrium diagram) is a diagram with T and composition as axes, showing the equilibrium constitution. The phase diagram of an alloy made of components A and B, for all combinations of T and X B, defines the A-B system. Binary systems have two components, ternary systems three, and so on.
This problem asks that we determine the phase mass fractions for the alloys and temperatures in Problem 9.8. (a) From Problem 9.8a, ε and η phases are present for a 90 wt% Zn-10 wt% Cu alloy at 400 °C, as represented in the portion of the Cu-Zn phase diagram shown below (at point A).
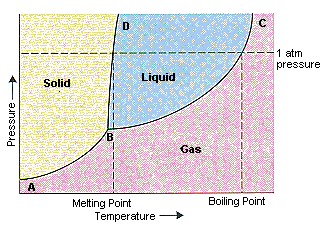
Examine the following phase diagram and determine what phase(s) exists at point a.
Examine the following phase diagram and determine what phases exists at point a. Examine the following phase diagram and identify the feature represented by point a. White fall 2013 10. Chem 1412 su06 exam 1. Solved by professors experts. Circuit elements that allow the flow of electrical current in only one direction. • Phase Diagrams Phase diagrams are graphs that give information on the equilibrium temperature and pressure for a particular compound. The equilibria occur for the solid- liquid plateau, liquid-vapor plateau and solid-vapor plateau. In this experiment, the phase diagram is shown for the solid-liquid equilibrium point, and varies from 100% ... Chapter 8 Phase Diagrams. (b) The interpretation of diagrams. Point a represents the vapor pressure of a mixture with liquid composition xA and b represents the composition of the vapor that is in equilibrium with the liquid at that pressure. Note that when two phases are in equilibrium, P = 2, so F’ = 1.
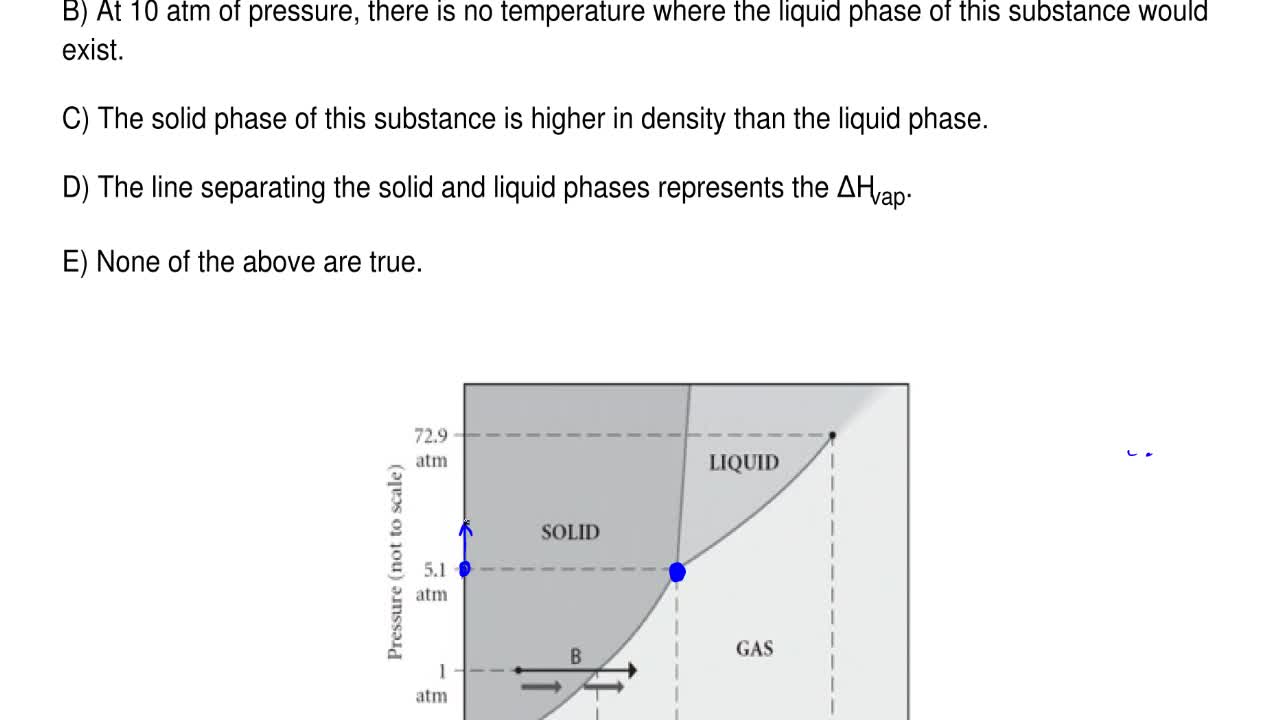
Examine the following phase diagram and determine what phase(s) exists at point a.. Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. A) Bo(s) has a lower density than Bo(l) B) the triple point for Bo is at a higher temperature than the melting point for Bo C) Bo changes from a solid to a liquid as one follows the line from C to D Examine the following phase diagram and determine what phase exists at point favapor liquidb. Phase Diagrams Of Pure Substances Octane is a component of fuel used in internal combustion engines. Examine the following phase diagram and determine what phase(s) exists at point a.. Examine the phase diagram for the substance bogusium bo and select ... 7) What is the phase (s, l, g) of a substance at 0.5 atm and 100 0 C? 8) What is the phase (s, l, g) of a substance at 1.5 atm and 50 oc? 9) What is the phase (s, l, g) of a substance at 1.5 atm and 200 oc? 10) What is the phase (s, l, g) of a substance at 1.5 atm and 800 oc? 11) What is the condition of the triple point of this substance? T= Phase Diagram. In this phase diagram, which is typical of most substances, the solid lines represent the phase boundaries. The green line marks the freezing point (or transition from liquid to solid), the blue line marks the boiling point (or transition from liquid to gas), and the red line shows the conditions under which a solid can be ...
Phase Diagram: In this phase diagram, which is typical of most substances, the solid lines represent the phase boundaries.The green line marks the freezing point (or transition from liquid to solid), the blue line marks the boiling point (or transition from liquid to gas), and the red line shows the conditions under which a solid can be converted directly to a gas (and vice-versa). Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. SEE QUESTION 10 Image A) Bo(s) has a lower density than Bo(l). B) The triple point for Bo is at a higher temperature than the melting point for Bo. C) Bo changes from a solid to a liquid as one follows the line from C to D. PHASE DIAGRAMS: Phase diagrams are another tool that we can use to determine the type of equilibration process and the equilibrium solution. In a phase diagram we graph y(t+1) as a function of y(t). We use a line of slope +1 which passes through the origin to help us see how the time path will evolve. The slope of the phase line
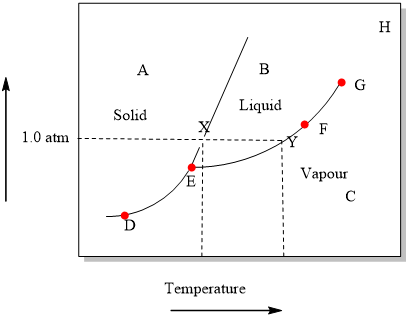
Examine the following phase diagram and determine what phase exists at point F. Examine the following phase diagram and determine what phase exists at point F. A)Vapor + Liquid. B)Vapor. C)Liquid. D)Solid. E)Supercritical fluid. Categories. 38. Examine the following phase diagram and identify the feature represented by point A. A. melting point B. critical point C. triple point D. sublimation point 39. What are the changes in phase going from points A to B to C to D A B D C T P A. melting, vaporization, deposition B. vaporization, freezing, sublimation Answer to examine the following phase diagram and determine what phase exists at point fa vapor liquidb vaporc liquidd. A the upper right region of the phase diagram is the liquid region while the lower right region is the region of gas b melting involves s l. Consider the following phase diagram and identify the process occurring as one goes ... Chapter 12-Consider the following phase diagram and identify the process occurring as one goes from point C to point D. Increasing temperature with a phase change from solid to vapor-Examine the following phase diagram and determine what phase exists at point F. Vapor-Which of the following intermolecular forces is the weakest? a) Dispersion forces, b) dipole-dipole interactions, c) dipole ...
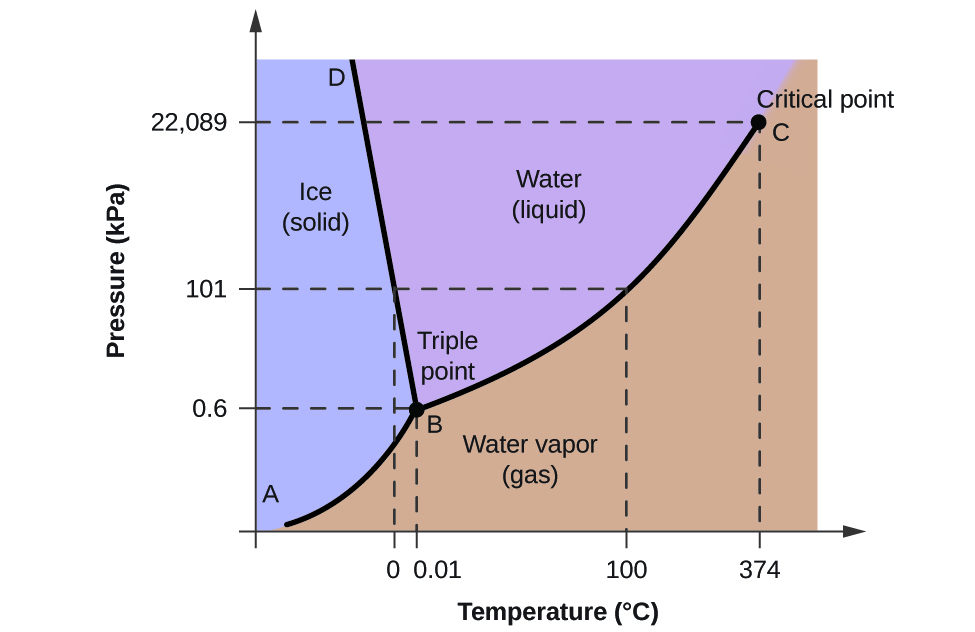
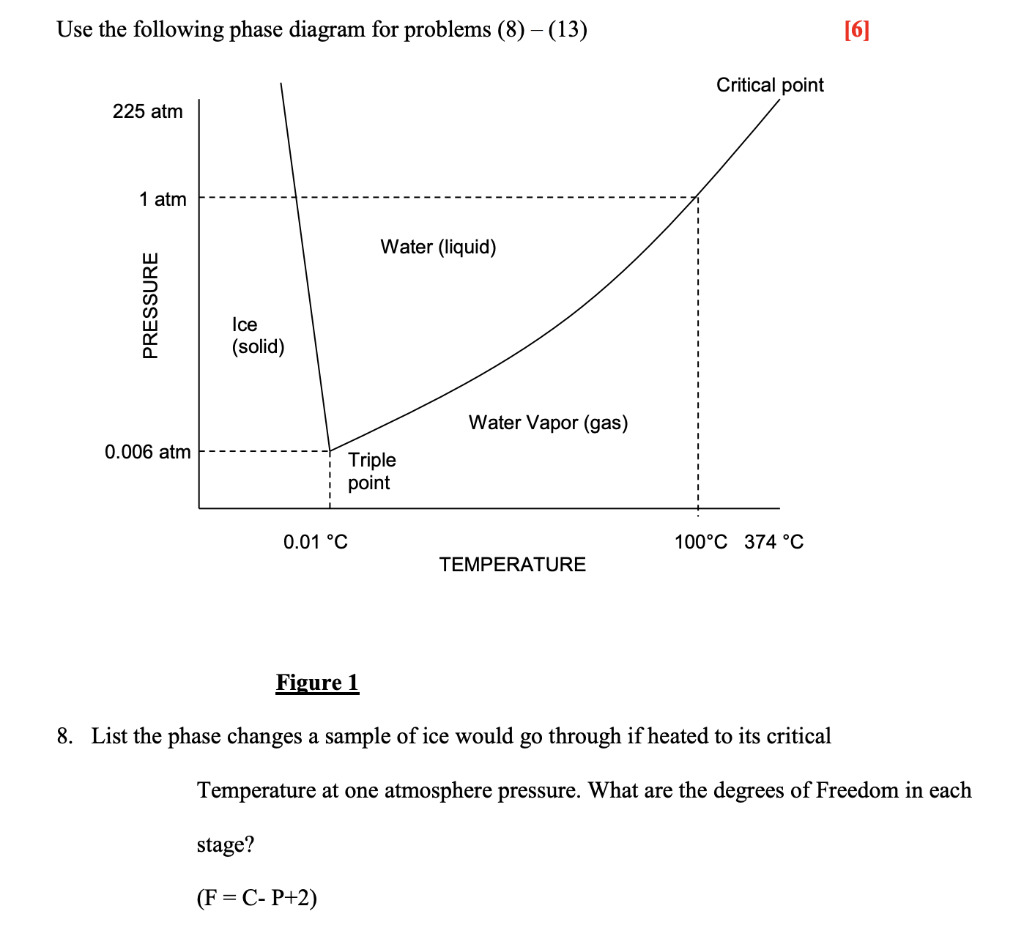
A phase diagram is a graphical representation of pressure and temperature of a material. Phase diagrams show the state of matter at a given pressure and temperature. They show the boundaries between phases and the processes that occur when the pressure and/or temperature is changed to cross these boundaries.
Use phase diagrams to identify stable phases at given temperatures and pressures, and to describe phase transitions resulting from changes in these properties. In the previous module, the variation of a liquid's equilibrium vapor pressure with temperature was described. Considering the definition of boiling point, plots of vapor pressure ...

Formation Of Salicylic Acid 4 4 Dipyridyl Cocrystals Based On The Ternary Phase Diagram Lee 2015 Chemical Engineering Amp Technology Wiley Online Library
Examine the following phase diagram and determine what phase exists at point F Vapor When the electron cloud of a molecule is easily distorted, the molecule has a high _____________.
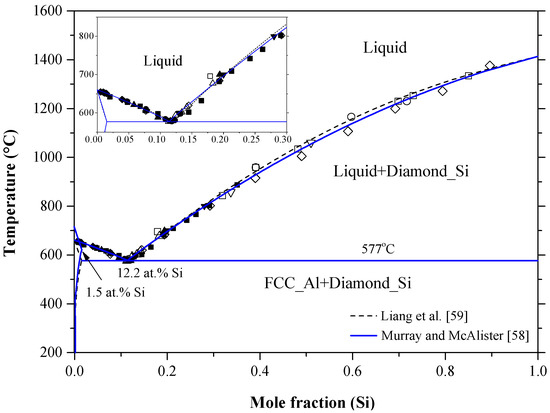
Materials Free Full Text Binary Phase Diagrams And Thermodynamic Properties Of Silicon And Essential Doping Elements Al As B Bi Ga In N P Sb And Tl Html
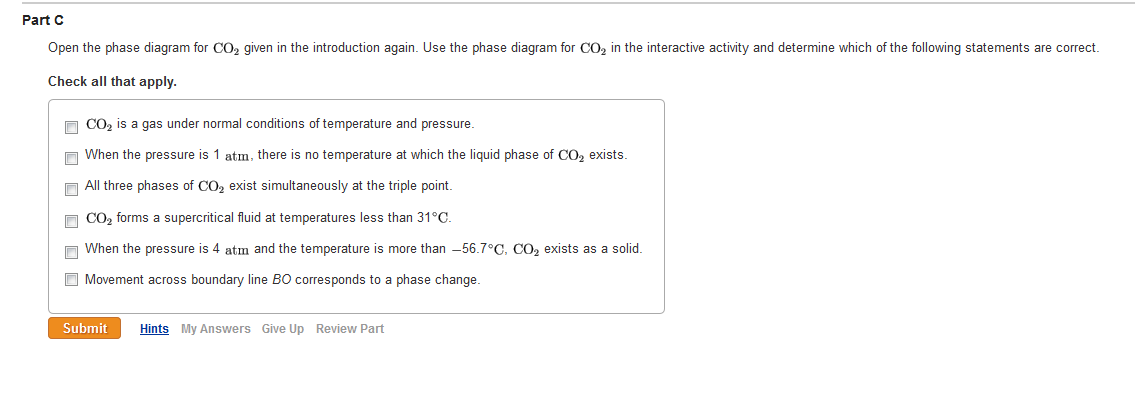
Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ...

Phase I Dose Escalation Single Centre Clinical Trial To Evaluate The Safety Of Infusion Of Memory T Cells As Adoptive Therapy In Covid 19 Release Eclinicalmedicine
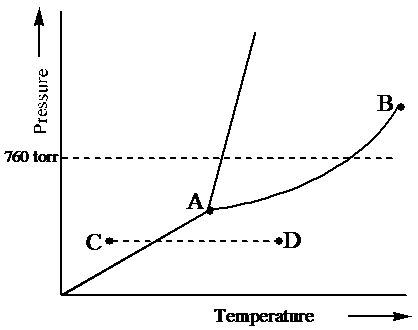
Examine the following phase diagram and determine what phase exists at point c. In the cases well be looking at on this page the phases will simply be the solid liquid or vapour gas states of a pure substance. What type of unit cell is this. Consider the following phase diagram and identify the process occurring as one goes from point c to point d.
Examine the following phase diagram and identify the feature represented by point A. A. melting point B. critical point C. triple point D. sublimation point E. boiling point 2. Examine the following phase diagram and determine what phase exists at point F. A. vapor + liquid B. vapor C. liquid D. solid E. supercritical fluid 1
Question. : 12) Examine the following phase diagram and identify the feature represented by point A. 760 torr Temperature A) melting point B) critical point C) triple point D) sublimation point E) boiling point 13) In hydrogen iodide are the most important intermolecular forces A) B) C) dipole-dipole forces London dispersion forces hydrogen ...
The phase portrait shares characteristics with that of a node. With only one eigenvector, it is a degenerated-looking node that is a cross between a node and a spiral point (see case 6 below). The trajectories either all diverge away from the critical point to infinite-distant away (when r > 0), or all converge to the critical point (when r < 0 ...
Assume that (1) α and β phases exist at the A and B extremities of the phase diagram, respectively; (2) the eutectic composition is 47 wt% B-53 wt% A; and (3) the composition of the β phase at the eutectic temperature is 92.6 wt% B-7.4 wt% A. Determine the composition of an alloy that will yield primary α
Examine the following phase diagram and determine what phase exists at point f. Examine the following phase diagram and determine. See question 10 image a bos has a lower density than bol. B london dispersion forces. The heat of vaporization for either is 2669 kjmol. Examine the following phase diagram and determine what phase exists at point f.
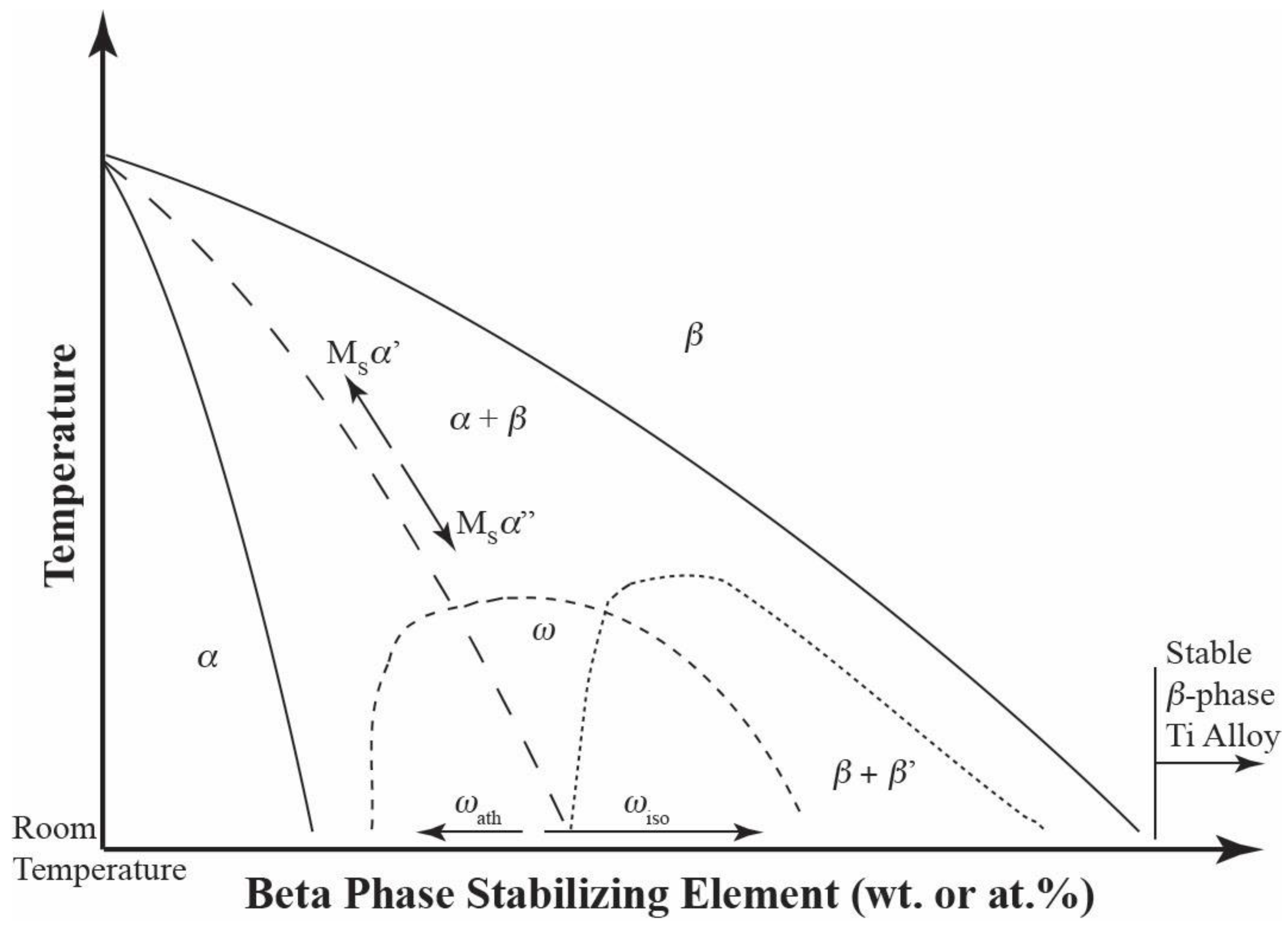
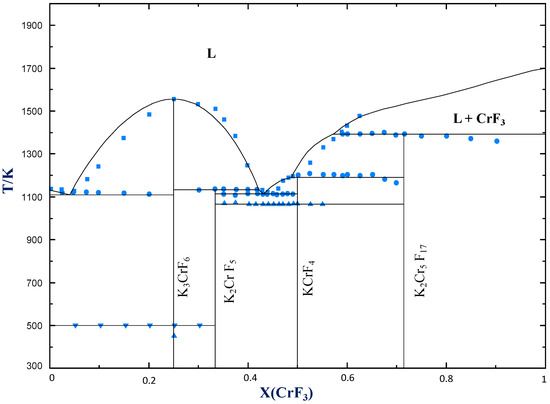
Peritectic point - The point on a phase diagram where a reaction takes place between a previously precipitated phase and the liquid to produce a new solid phase. When this point is reached, the temperature must remain constant until the reaction has run to completion. A peritectic is also an invariant point. Intermediate compound - A phase that ...
Chemistry questions and answers. Examine the following phase diagram and determine what phase exists at point 760 som Temperature A) supercritical fluid B) liquid C) vapor+liquid D) vapor E) solid The phase diagram of a substance i s given below. This substance is a at 25°C and 1.0 atm. 1.5 P (atm) 1.0 0.5T -10 0 10 20 30 40 50 60 70 T ('C) A ...
Below is shown the titanium-copper phase diagram (Figure 9.37). There is one eutectic on this phase diagram, which exists at about 51 wt% Cu-49 wt% Ti and 960°C. Its reaction upon cooling is 2 L! TiCu + TiCu There is one eutectoid for this system. It exists at about 7.5 wt% Cu-92.5 wt% Ti and 790°C. This reaction upon cooling is ! " # + Ti2Cu
(a) That portion of the Cu-Zn phase diagram (Figure 9.19) that pertains to this problem is shown below; the point labeled "A" represents the 90 wt% Zn-10 wt% Cu composition at 400°C. As may be noted, point A lies within the ε and η phase field. A tie line has been constructed at 400°C; its
Chapter 8 Phase Diagrams. (b) The interpretation of diagrams. Point a represents the vapor pressure of a mixture with liquid composition xA and b represents the composition of the vapor that is in equilibrium with the liquid at that pressure. Note that when two phases are in equilibrium, P = 2, so F’ = 1.
• Phase Diagrams Phase diagrams are graphs that give information on the equilibrium temperature and pressure for a particular compound. The equilibria occur for the solid- liquid plateau, liquid-vapor plateau and solid-vapor plateau. In this experiment, the phase diagram is shown for the solid-liquid equilibrium point, and varies from 100% ...
Examine the following phase diagram and determine what phases exists at point a. Examine the following phase diagram and identify the feature represented by point a. White fall 2013 10. Chem 1412 su06 exam 1. Solved by professors experts. Circuit elements that allow the flow of electrical current in only one direction.
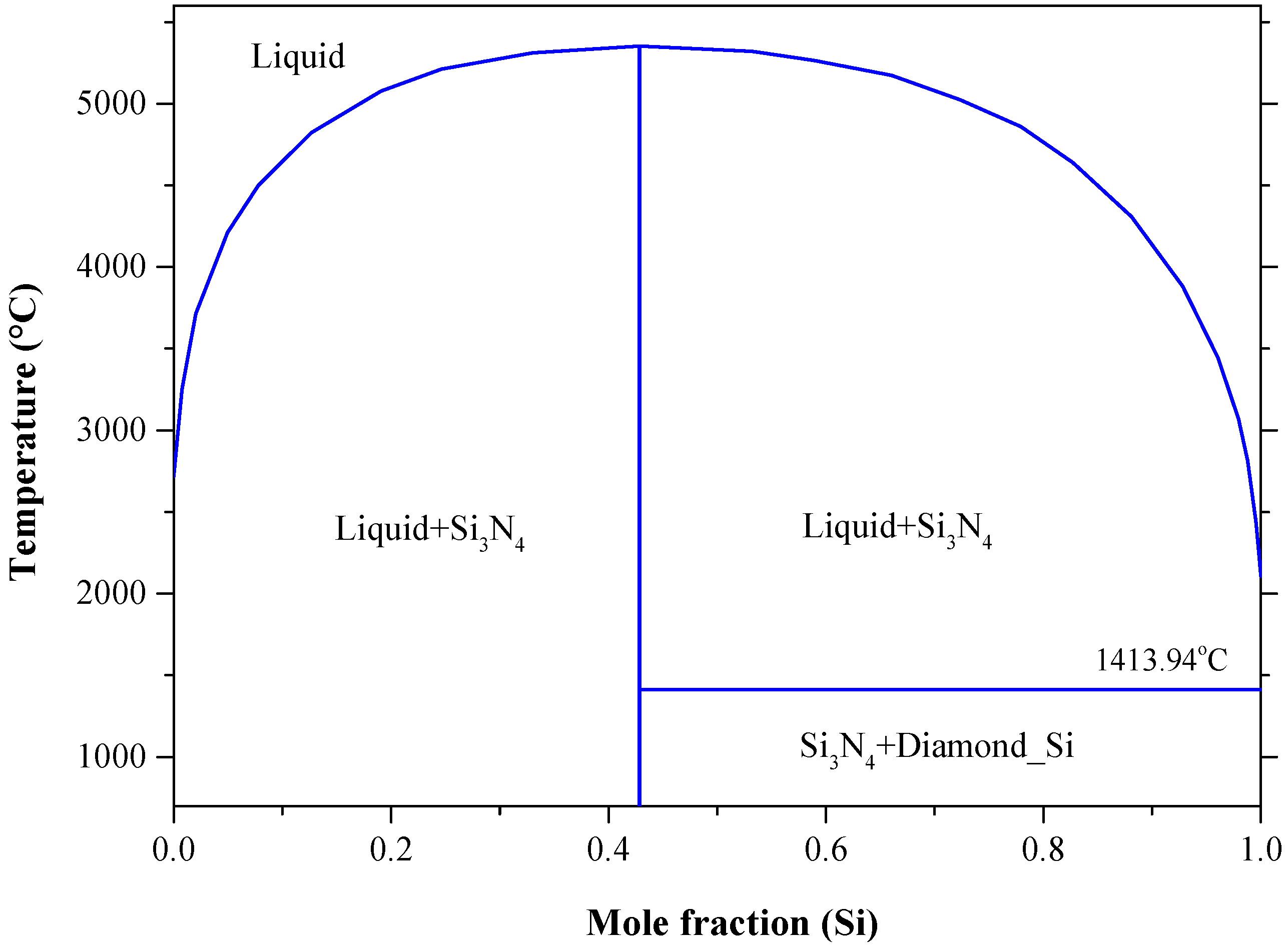
Materials Free Full Text Binary Phase Diagrams And Thermodynamic Properties Of Silicon And Essential Doping Elements Al As B Bi Ga In N P Sb And Tl Html
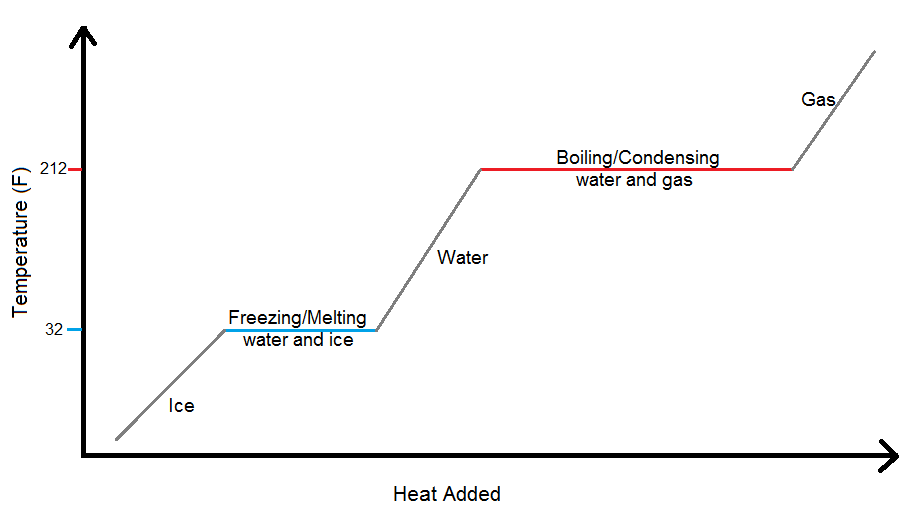
How Would You Use The Phase Diagram Of Water To Explain Why Ice At The Bottom Of A Glacier Can Melt When The Rest Of The Glacier Which Is The Same Temperature
















0 Response to "39 examine the following phase diagram and determine what phase(s) exists at point a."
Post a Comment