38 orbital diagram of silicon
Si orbital Diagram. electron configuration of silicon si orbital diagram orbital diagram electron configuration and the noble gas notation for a silicon si atom electron configuration for silicon si how to write the electron configuration for silicon si since 1s can only hold two electrons the next 2 electrons for silicon go in the 2s orbital. Taking that into consideration: s sublevel has 1 orbital, and therefore can hold max 2 electrons p sublevel has 3 orbitals, and can hold max 6 electrons d sublevel has 5 orbitals, and can hold max 10 electrons When you fill up the orbitals from the lowest energy starting from 1s, you'll notice that all 14 electrons of Silicon are filled up when ...
Silicon has an electron configuration of 1s2 2s2 2p6 3s2 3p2. Using the noble gas notation, the electron configuration of silicon can be denoted by Ne 3s 2 3p 2. In the periodic table of elements, silicon is represented by the chemical symbol Si, atomic number 14 and relative atomic mass of 28.085.

Orbital diagram of silicon
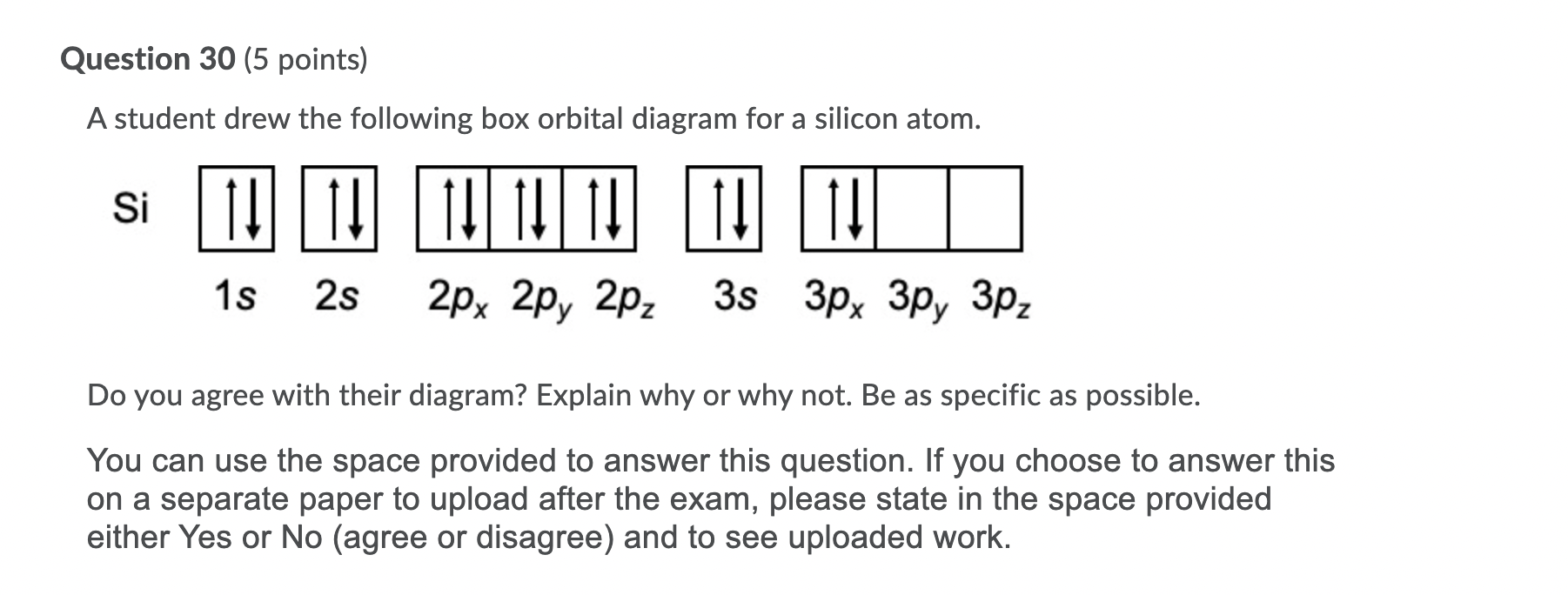
Question: examine the orbital diagram for the ground state electron configuration of silicon choose best dxplanataion for why this orbital diagram is incorrect foe the ground state of silicon. This problem has been solved! See the answer See the answer See the answer done loading. 1. Orbital Filling Diagram 02 Ex. 2, Electron Configuration 02 Ex. (gives the most information) Is (quicker to draw than orbital filling diagrams) Dot Pb 3. Electron Dot shows only the valence (outer energy level) electrons Oxygen atom Ex. 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following ... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
Orbital diagram of silicon. What is the orbital notation for silicon? Its hard to put this on a screen with a regular key board. But the 1's at the top represent arrows in the pared up arrows imagine the first one pointing ... An orbital-filling diagram shows the number of electrons in each orbital, which are shown in order of energy. The placement of electrons in orbitals follows a certain set of rules. Lower energy subshells fill before higher energy subshells. The order of filling is 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p. Orbital Diagram For Arsenic. Because the 4p section has 3 orbitals, but Arsenic ends with 4p3. It'll want to leave as few orbitals empty, so you have three arrows pointing up. The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in itsoutermost shell. schematron.org! Naim was happy, Naim felt safe, he crawled through a duct between decks and found the issue Mick had chirped at him, with his deft claws he unhooked a data cable unwound the fixing piece and carefully stripped back the clear wires, Naim did not know why these wires were clear, some had liquid in them others seemed to be filled with light. It didn't matter to him, as long as the Goddess was happy so was Naim, as he worked, fitting each strand into a connector. It amused him that he could recogni...
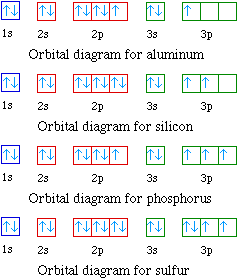
By Hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. I hope you enjoy part 2 __________________________________ *A very small percentage of these species have the right combination to create technic societies. But when we deal with the tremendous numbers of planets that exist in your standard spiral galaxy, this still equates to hundreds of thousands of technic species that inevitably seek to explore the limitless void of their home galaxy. Some species evolve on oxidizing worlds and use oxygen as their primary electron acceptor, carbon chains fo... This time we have collected a handy of orbital diagrams with various types in high definition! There are alkali, argon, electron, nitrogen, phosphorus, silicon, and sulfur orbital diagrams that you can save for free. Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams. 9.7 The orbital diagram below presents the final step in the CQ formation of hybrid orbitals by a silicon atom. (a) Do you think one or more electrons have been promoted? Why or why not? (b) What type of hybrid orbitals are being produced in this hybridization? [Section 9.5] 9.8 Consider the hydrocarbon drawn below. (a) What is the
I live smack-dab in the middle of Nowhere, South Carolina, and I thought the moment I found a real, actual meteor in the woods around my house was going to be the coolest moment in my entire life. If I could put it back, I would. The crater was around three feet across, and I can’t really say if I was more or less excited when I saw that it was a piece of wreckage and not an actual space rock. The black box was an armored canister around the size of a soda can, with the NASA logo emblazoned hel... Orbital Diagram, electron configuration, and the noble gas notation for a silicon (Si) atom. Orbital diagrams are pictorial descriptions of the electrons in an atom. Three guidelines are helpful in forming orbital diagrams. In accordance with the Auf Bau Precept, every electron occupies the bottom power orbital. You leap up somewhat bit in power and we get the 2s orbital that make it the 2p sublevel. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to represent the electrons in ...
The Orbital Diagram for Silicon: The orbital diagram for an element shows the electron distribution of the electrons, and the correct pairing of electrons with respect to electron spin.

17 Write Ground State Electron Configuration And Orbital Box Diagram For Silicon State The Number Of Homeworklib
So I need someone to check some of these, so I might crosspost this to other subreddits, if you know any, please do. Or if you are an expert yourself, please correct me if there's any mistakes. But I did watch Dr. Stone in an *Anime Streaming website*, I posted some interesting comments in the discussions of Dr. Stone Episodes. I will post them in a chronological order with the matching episodes. Although, I think it's a bad idea to post this in a whole one post. Because no one gonna read it t...
Figure 12.21 The Molecular Orbital Energy-Level Diagram for a Linear Arrangement of n Atoms, Each of Which Contains a Singly Occupied s Orbital. This is the same diagram as Figure 9.35 "Bonding in Ozone", with the addition of the far right-hand portion, corresponding to n = 30 and n = ∞. As n becomes very large, the energy separation between adjacent levels becomes so small that a single ...
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid and semiconductor.It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive. Because of its high chemical affinity for oxygen ...
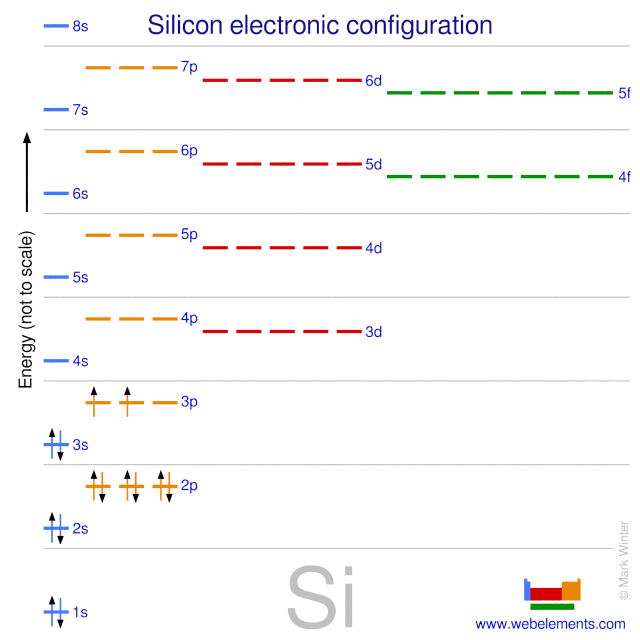
Silicon is composed of 14 electrons, 14 protons, and (in most cases) 14 neutrons. In its ground state, silicon has two electrons in the n = 1 energy level, eight in the n = 2 energy level, and four in the n = 3 energy level, as shown on the energy diagram to the left.

Electrons In Atoms Basic Properties Of Waves Amplitude L Amplitude Is The Maximum Distance The Particles Of The Medium Carrying The Wave Move Away From Ppt Download
​ EDIT/ADDITION: [Patches of Grass Telling the Future? This Seals the Deal!](https://www.reddit.com/r/FortNiteBR/comments/9d2f7d/thanks_to_you_guys_ive_made_a_map_on_where_the/) Part 1/3: Well, the hints are all there you just have to piece it together. The hippie is what cracked this case wide open for ol' Private Investigator Monterhey. Let me lay it out for you: Hippies + Cubes = Sugarcubes Sugarcubes + Hippies =LSD. Confused? History lesson. Please welcome Professor Tripp is her...
Hey, Vsauce. Michael here. This is Earth as seen from Saturn. That is us right there. And if you look closely, ok, see this little protuberance? That's the Moon. This image was taken by the Cassini spacecraft on July 19th, 2013 at 21:27 Coordinated Universal Time. The thing is, NASA gave the public advanced warning of when it would be taken, which means that this image of Earth was the first ever taken from space that some people on Earth were actually posing for. Our planet looks so small, ins...
Nov 01, 2021 · Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15: Orbital diagram of Phosphorus ...
Silicon orbital diagram. Show the orbital-filling diagram for S sulfur. It is a hard brittle crystalline solid with a blue-grey metallic lustre and is a tetravalent metalloid and semiconductorIt is a member of group 14 in the periodic table. Also the crystalline form is used in semiconductors. Three rules are useful in forming orbital diagrams.
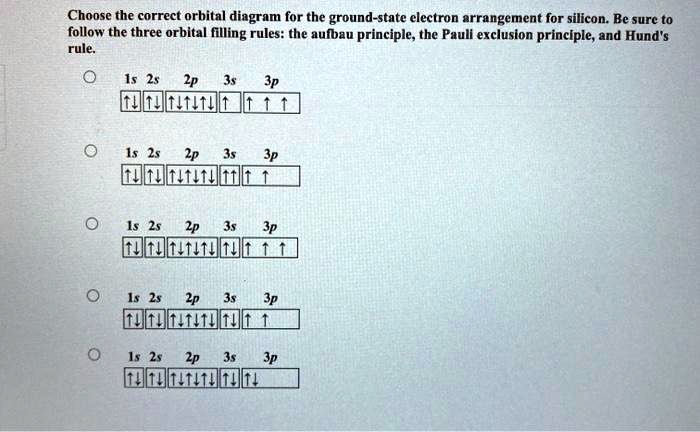
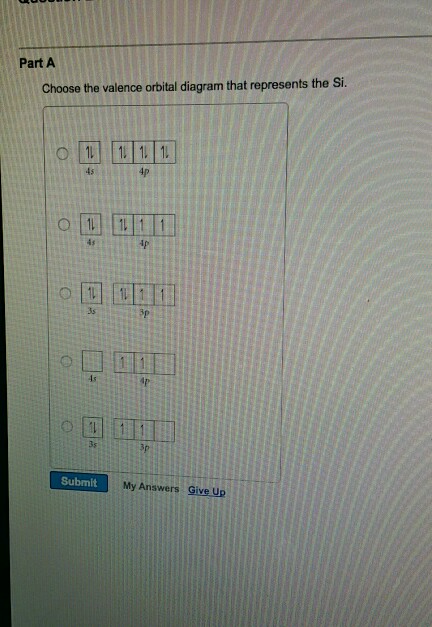
Solved Choose The Correct Orbital Diagram For The Ground State Electron Arrangement For Silicon Be Sure To Follow The Three Orbital Filling Rules The Aufbau Principle The Pauli Exclusion Princlple And Hund Rule Wdmtit
The space shuttle is a reusable winged machine capable of controlled flight in the Earth's atmosphere as well as in space. The idea of a rocket that could do that was at first considered crazy. But as well-known writer Arthur C. Clarke said, "every idea that people stop laughing will be realized in fifty years." "Shuttle buses for several dozen passengers. Transporters with a load capacity of 300-500 t. Maximum reachable height 500 km above the Earth. Twenty, fifty, one hundred, five hundred s...
Oxygen electron configuration is 1s 2 2s 2 2p 4.The period of oxygen is 2 and it is a p-block element. This article gives an idea about the electron configuration of oxygen(O) and orbital diagram, period and groups, valency and valence electrons of oxygen, bond formation, compound formation, application of different principles.
[Wu-Tang Clan](https://media1.fdncms.com/metrotimes/imager/u/original/21756159/wu-tangclan-723a8c68e7_1_.jpg) rap | hiphop | anit nothing to fuck with [Similar Artists](https://www.music-map.com/wu-2dtang+clan.html) ----- Emerging in 1993, when Dr. Dre's G-funk had overtaken the hip-hop world, the Staten Island, New York-based Wu-Tang Clan proved to be the most revolutionary rap group of the mid-'90s -- and only partially because of their music. Turning the standard concept of a hip-hop cre...
Science Fiction Or Science Fact Silicon Based Alien Life Treknews Net Your Daily Dose Of Star Trek News And Opinion
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below)
Hi there are a few questions I have about my homework 1. Why are some elements Diamagnetic when (unless im just not understanding something) they shouldn't be? Specifically, Silicon (Si) is said to be Diamagnetic but the Orbital Diagram of Si has unpaired electrons? (this is before I've learnt about (anti-)Ferromagnetic elements, so that doesn't matter for me) 2. How do I draw an excited state of an element (orbital diagram)? The homework says "Your only restrictions are that you have to draw a...
Therefore the Silicon electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 2.The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds.
Oxidation States, ±4. Electrons Per Shell, 2 8 4. Electron Configuration, [Ne] 3s2 3p2. 1s2 2s2 2p6 3s2 3p2. Orbital Diagram.
Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11.
Sodium electron configuration is 1s 2 2s 2 2p 6 3s 1.The symbol for sodium is ‘Na’. The period of sodium is 3 and it is a s-block element. This article gives an idea about the electron configuration of sodium(Ne) and orbital diagrams, period and groups, valency and valence electrons of sodium, bond formation, compound formation, application of different principles.
Silicon Orbital Diagram Orbit diagram consists of a pair of electrons of the atom in the box i.e. Orbit diagram helps to define the ground-state electron configuration is an easy form. That is one box contains 2 electrons. And for silicon, there will be 7 box representations for 14 electrons in a pair.

Scielo Brasil A Brief Introduction To Molecular Orbital Theory Of Simple Polyatomic Molecules For Undergraduate Chemistry Students A Brief Introduction To Molecular Orbital Theory Of Simple Polyatomic Molecules For Undergraduate
How Many Valence Electrons are in Silicon. There are 4 electrons in the outer shell of Silicon so the number of valence electrons in silicon is 4. Silicon Orbital Diagram. Orbit diagram consists of a pair of electrons of the atom in the box i.e. Orbit diagram helps to define the ground-state electron configuration is an easy form.
Hey, Vsauce. Michael here. This is Earth as seen from Saturn. That is us right there. And if you look closely, ok, see this little protuberance? That's the Moon. This image was taken by the Cassini spacecraft on July 19th, 2013 at 21:27 Coordinated Universal Time. The thing is, NASA gave the public advanced warning of when it would be taken, which means that this image of Earth was the first ever taken from space that some people on Earth were actually posing for. Our planet looks so small, ins...
Silicon (Si) is a crystalline blue-grey solid with a metallic appearance. It is a metalloid that has the atomic number 14 in the periodic table. It is in Group 14 of the periodic table. It has the symbol Si.
(Recall from Section 5.3B that two electrons in an orbital spin in opposite directions on their axes.) Therefore, if an orbital contains two electrons, its box will contain two arrows, one pointing up and the other down. Using a box diagram, we show the electron configuration of nitrogen as: Notice that the 2p electrons are shown as
In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital.
In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two ...
Silicon atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. Check out the blackboard. That box on the left has all of the information you need to know about one element. It tells you the mass of one atom, how many ...
What is silicon orbital configuration? In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital. Therefore the Silicon electron configuration will be 1s22s22p63s23p2.

Table Element Orbital Filing Diagram Election Configuration Electron Dot Dagram A Boron B Silicon C Sulfur Homeworklib
Silicon") and Its Use the orbital diagram to find the third and eighth electrons. PROBLEM: Write a set of .Which ground-state atom has an electron configuration described by the following orbital diagram? A antimony B germanium C indium D lead E tin%(1).
Hey, Vsauce. Michael here. This is Earth as seen from Saturn. That is us right there. And if you look closely, ok, see this little protuberance? That's the Moon. This image was taken by the Cassini spacecraft on July 19th, 2013 at 21:27 Coordinated Universal Time. The thing is, NASA gave the public advanced warning of when it would be taken, which means that this image of Earth was the first ever taken from space that some people on Earth were actually posing for. Our planet looks so small, ins...
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...

Aiims Pedia 190 Which Of The Following Orbital Box Diagrams Represents Silicon Which Has 14 Electrons Facebook
1. Orbital Filling Diagram 02 Ex. 2, Electron Configuration 02 Ex. (gives the most information) Is (quicker to draw than orbital filling diagrams) Dot Pb 3. Electron Dot shows only the valence (outer energy level) electrons Oxygen atom Ex. 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following ...
Question: examine the orbital diagram for the ground state electron configuration of silicon choose best dxplanataion for why this orbital diagram is incorrect foe the ground state of silicon. This problem has been solved! See the answer See the answer See the answer done loading.
















0 Response to "38 orbital diagram of silicon"
Post a Comment