37 thin layer chromatography diagram
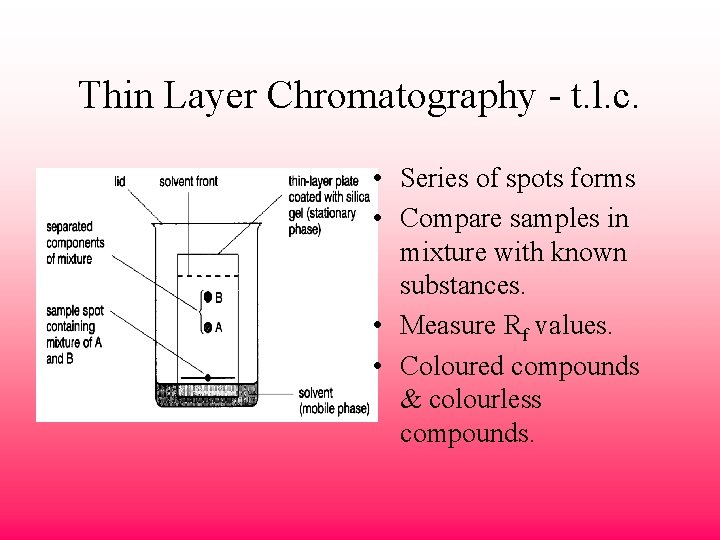
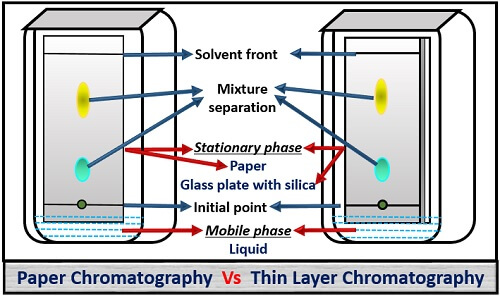
What is Thin Layer Chromatography?Thin-layer chromatography is a technique in which the analytes of a mixture are separated by differential migration through the stationary phase. TLC is performed on glass, aluminum or plastic sheets. Thin-layer chromatography (TLC) has developed into a very sophisticated technique for identification of compounds and for determination of the presence of trace impurities. Since it was one of the earliest chromatographic techniques, a huge array of TLC-based tests is available and pharmacopoeial...
Thin Layer Chromatography. Chromatography Overview Chromatography is the general name for a variety of techniques used in the physical separation of mixtures. Chromatography separates compounds based on their differing affinities for a medium known as the stationary phase.

Thin layer chromatography diagram
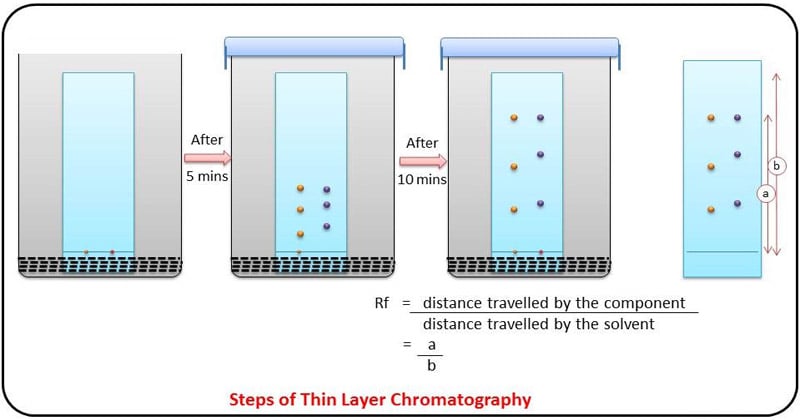
1. Introduction to TLC (Thin-Layer Chromatography). 4. Performing a Thin-Layer Chromatography (TLC) Analysis. ChemistryConnected. Using thin layer chromatography to identify compounds. Suppose you had a mixture of amino acids and wanted to find out which particular amino acids the The left-hand diagram shows the plate after the solvent front has almost reached the top. The spots are still invisible. The second diagram shows... I've seen a couple posts asking about tested lab techniques, so I figured we could compile a master list. Feel free to comment on what I should add/remove/emphasize. If you have any good videos or resources I can add it to the list as well. I know some on here aren't as high yield but I figure anything that could be tested on is worth listing. #SEPARATIONS AND PURIFICATIONS * extraction * filtration * distillation * simple distillation * fractional distillation * vacuum distillation * recrys...
Thin layer chromatography diagram. Thin-layer chromatography (TLC) is used for identifying compounds and determining their purity. The most common adsorbent used is silica gel. Compounds should separate the same on an alumina plate as on an alumina column, and column chromatography using alumina is still very popular. Thin-layer chromatography (TLC) is a very commonly used technique in synthetic chemistry for identifying compounds, determining their purity and following the progress of a reaction. It also permits the optimization of the solvent system for a given separation problem. In comparison with column... Thin-layer chromatography (TLC) is a fast, easy-to-use, and highly versatile separation technique for qualitative and quantitative analysis, it is ideal for rapid identification of ingredients, screening and reaction monitoring. Its high matrix tolerance and the possibility to separate many samples in parallel... With 3 days left until I test, I can’t help but hope 9/4’s MCAT has everything I feel strong on. Was also curious what other people hoped for on theirs. C/P: Dream - batteries/redox reactions - stereochemistry - stoichiometry/molar ratios - substitution and elimination reactions - kinematic and force/energy - gas laws - periodic trends - radioactive decay/types - thin layer chromatography and separation techniques - free energy, enthalpy, entropy Nightmare - obscure organic chemistry ...
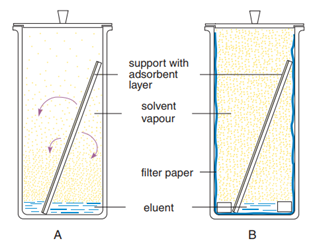
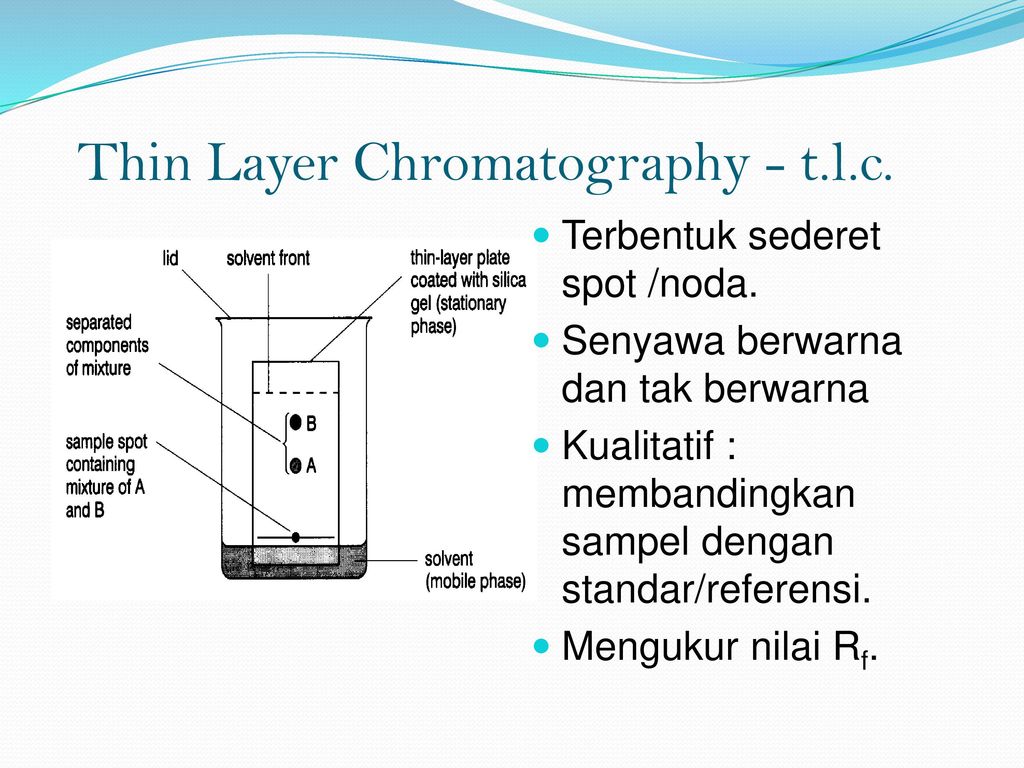
Thin Layer Chromatography, or TLC, is the most basic technique for synthetic chemists. Learn how to master it from the very beginning! Thin Layer Chromatography is a cheap, quick and easy technique to separate components of a mixture. It is used by synthetic chemists to monitor chemical... Introduction to Thin Layer Chromatography: Chromatography is used to separate mixtures of substances into their components. All forms of chromatography work on the same principle. They all have a stationary phase (a solid, or a liquid supported on a solid) and a mobile phase (a liquid or a gas). Thin-layer chromatography is a separation technique that involves several steps. First, a solution made from a sample is applied to a coated plate. The carrier solvent of the sample solution evaporates and deposits the sample in a small spot or zone at the origin of the plate. INTRODUCTION: Thin layer chromatography (TLC) is a chromatography technique used to separate mixtures. Chromatography was discovered by M. Tswett in 1906.Thin layer chromatography is performed on a sheet of glass, plastic, or aluminum foil...
Thin Layer Chromatography. Table of Content. Definition Principle Diagram Procedure Applications FAQs. Thin Layer Chromatography Applications. The qualitative testing of Various medicines such as sedatives, local anaesthetics, anticonvulsant tranquilisers, analgesics, antihistamines, steroids... Thin-layer chromatography. TLC, particularly in its HPTLC version, can be considered as a (less expensive) alternative to LC for the purification and prefractionation of lipid extracts prior to gas chromatographic or radioligand assays of vitamin D metabolites in biological materials. Thin layer chromatography (TLC) is a method for analyzing mixtures by separating the compounds in the mixture based on polarity. TLC can be used to help determine the number of components in a mixture, the identity of compounds, and the purity of a compound. Thin-layer chromatography as practiced today seems to exist in two forms. Some scientists consider TLC to be a qualitative separation tool for simple mixtures where speed, low cost, and simplicity are its virtues. Others regard TLC as a powerful separation tool for the quantitative analysis of complex...
Thin layer chromatography has wide field of applications that include pharmaceuticals, food, cosmetics and phytochemistry.
Thin-Layer Chromatography: A Laboratory Handbook Egon Stahl (auth.)|Prof. Chromatographic Fingerprint Analysis of Herbal Medicines Volume III: Thin-layer and High Performance Liquid Chromatography of Chinese Drugs.
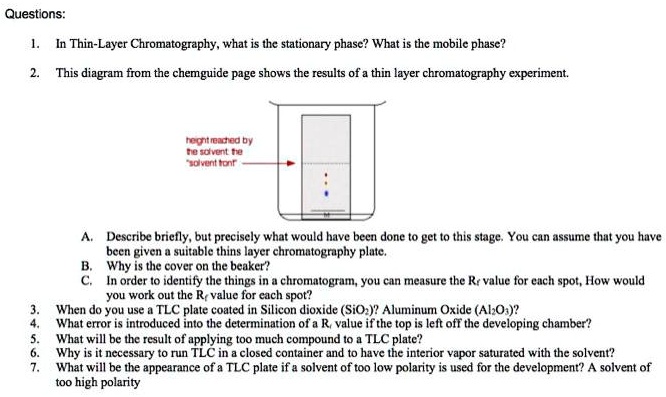
Thin Layer Chromatography (TLC) is a solid-liquid technique in which the two phases are a solid (stationary phase) and a liquid (moving phase). Solids most commonly used in chromatography are silica gel (SiO2 x H2O) and alumina (Al2O3 x H2O). Both of these adsorbents are polar, but alumina...
Thin-layer chromatography (TLC) is a chromatography technique used to separate non-volatile mixtures. Thin-layer chromatography is performed on a sheet of an inert substrate such as glass, plastic, or aluminium foil, which is coated with a thin layer of adsorbent material, usually silica gel...
TLC chromatography or thin layer chromatography is an analytical technique for easy identification of photo-constituents. TLC or Thin Layer Chromatography. TLC is a type of planar chromatography. Researchers routinely use it in the field of phytochemicals, biochemistry, and so...
Hey guys! I am trying to compile a list of all of the lab techniques/separation techniques that could appear on the MCAT. I think everything here is correct, but I would love feedback if I have messed up/written something wrong! If you can think of any other techniques that I should include here, please let me know and I can edit it! I also have a google doc that includes pictures/diagrams of each technique, so I'll include the link in the comments :). I hope at least a few people find this usef...
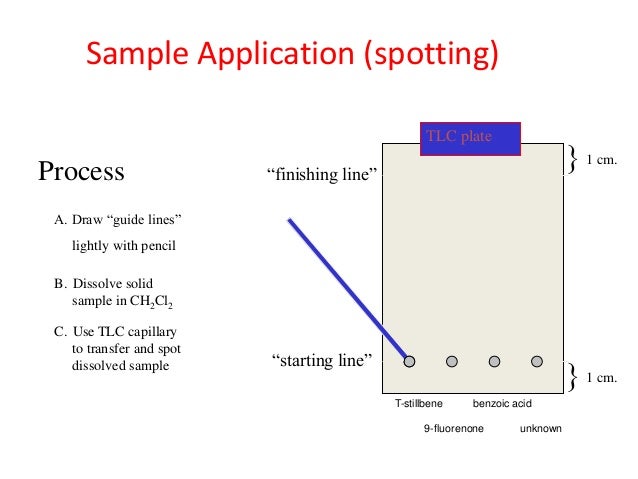
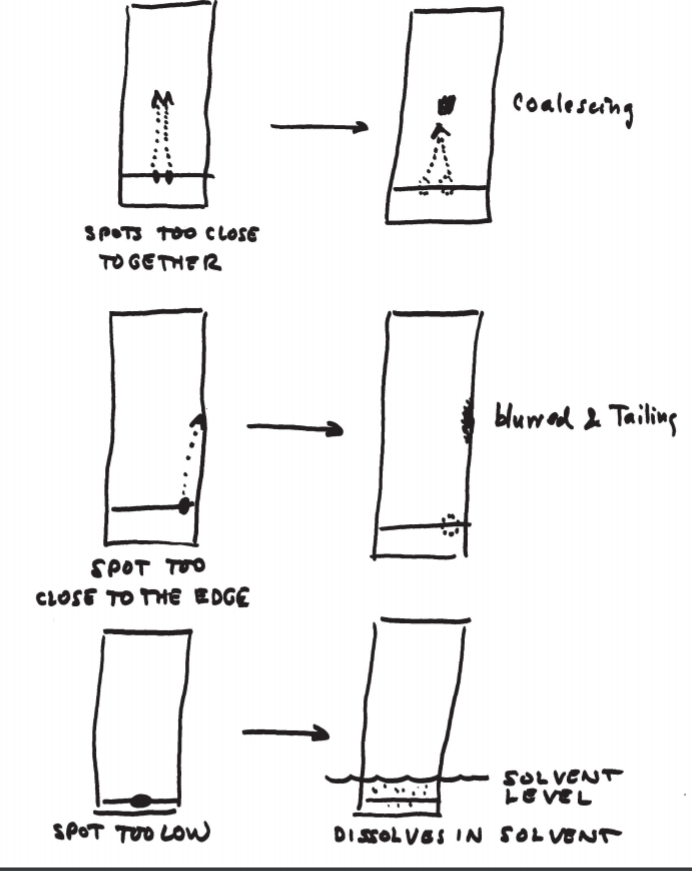
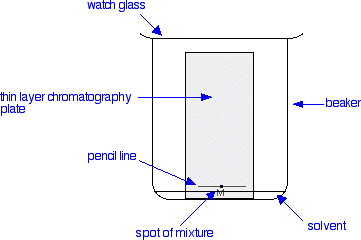
Procedure of Thin layer chromatography. Step I: Plate preparation Thin layer may be prepared by pouring, dipping, spraying or spreading the adsorbent slurry over the plate. Generally, the best procedure for the preparation of uniform layers or films is the use of the commercial spreader.
Thin-layer chromatography Principle The Principle of Thin layer chromatography is based on the separation of two or more components from a mixture by Running a mobile phase through a Stationary phase This can be Liquid-Liquid, Solid-Liquid, and Ga...
In Thin Layer Chromatography ("TLC"), a liquid solution is directly applied to a solid adsorbent. Capillary action draws a developing solvent up the TLC plate. As this solvent passes through the spot, the mixture will be dissolved and will begin to move with the solvent front.
Thin Layer Chromatography is the separation or identification of a mixture of components into individual components by using finely divided adsorbent Thin-layer chromatography is performed on a sheet of glass, plastic, or aluminium foil, which is coated with a thin layer of adsorbent material...
Thin layer chromatography (TLC) is an affinity-based method used to separate compounds in a mixture. TLC is a highly versatile separation method that is widely used for both qualitative and quantitative sample analysis. TLC can be used to analyze virtually any substance class, including...
Thin layer chromatography plate or TLC plate is commercially available. The chemical name of silica gel is silicon dioxide (silica) where silicon is attached with oxygen by covalent bond in a giant structure. TLC plate is prepared by mixing the silica gel with calcium sulfate and water.
Thin layer chromatography (TLC) is a quick, sensitive, and inexpensive technique used to determine the number of components in a mixture, verify the identity and purity of a compound, monitor the progress of a reaction, determine the solvent composition for preparative separations, and analyze the...
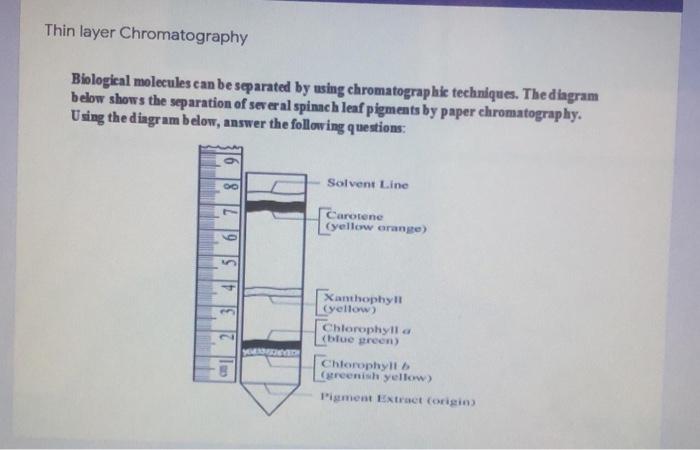
Learn about thin layer chromatography topic of chemistry in details explained by subject experts on vedantu.com. Their nature or character is identified using suitable detection techniques. Let's have a look at the thin layer chromatography diagram.
Thin layer chromatography (TLC) is a chromatographic technique used to separate the components of a mixture using a thin stationary phase supported by an inert backing. It may be performed on the …
I've seen a couple posts asking about tested lab techniques, so I figured we could compile a master list. Feel free to comment on what I should add/remove/emphasize. If you have any good videos or resources I can add it to the list as well. I know some on here aren't as high yield but I figure anything that could be tested on is worth listing. #SEPARATIONS AND PURIFICATIONS * extraction * filtration * distillation * simple distillation * fractional distillation * vacuum distillation * recrys...
Using thin layer chromatography to identify compounds. Suppose you had a mixture of amino acids and wanted to find out which particular amino acids the The left-hand diagram shows the plate after the solvent front has almost reached the top. The spots are still invisible. The second diagram shows...
1. Introduction to TLC (Thin-Layer Chromatography). 4. Performing a Thin-Layer Chromatography (TLC) Analysis. ChemistryConnected.



















0 Response to "37 thin layer chromatography diagram"
Post a Comment